United States
Thank you for contacting us; we’ll be in touch shortly.
Serialized Product Intelligence: The Name Says It All
Learn how Serialized Product Intelligence automatically analyzes serialization events to enable fast queries and visual reports that can be easily…

No More Serialization Surprises: Proactive Monitoring with Serialized Product Intelligence
Learn how Serialized Product Intelligence gives you visibility into your serialized operations, so can respond to data requests and resolve issues…

CPL and Serialized Product Intelligence: Leveraging Data for Business Value
CPL's Daryl Chin on how SPI helps him respond to customer inquiries, release batches more quickly, and invoice faster.

SCMiND
Partnering for Success: SCMiND President on the Advantages of TraceLink MINT Over P2P Integration
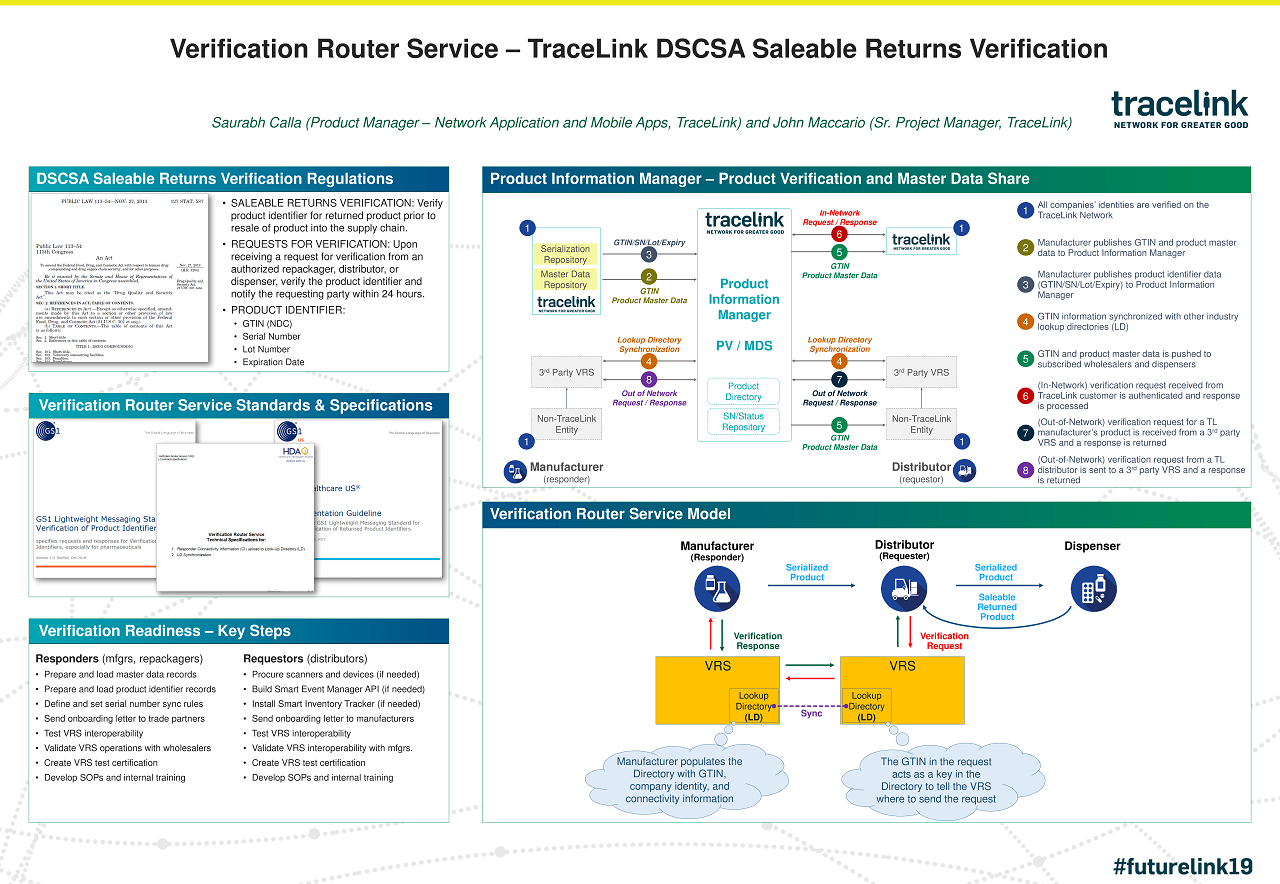
Case Study: TraceLink | Verification Router Service - TraceLink DSCSA Saleable Returns Verification
TraceLink helps customers meet DSCSA saleable returns verification requirements via the Verification Router Service model. See how.

Case Study: CPL | The CMO Serialization Perspective—Utilizing a Standardized Approach for Efficient Partner Onboarding
See how contract manufacturer Contract Pharmaceuticals Limited implemented a 3-step process for smooth pharmaceutical partner onboarding.

Case Study: TraceLink | FDA Pilot - Product Traceability Under 2023 DSCSA Regulations - A Business Process-Led Design for a Blockchain Network
TraceLink's breakthrough blockchain solution, Trace Histories, can help pharma customers comply with US DSCSA regulations that go into effect in 2023.
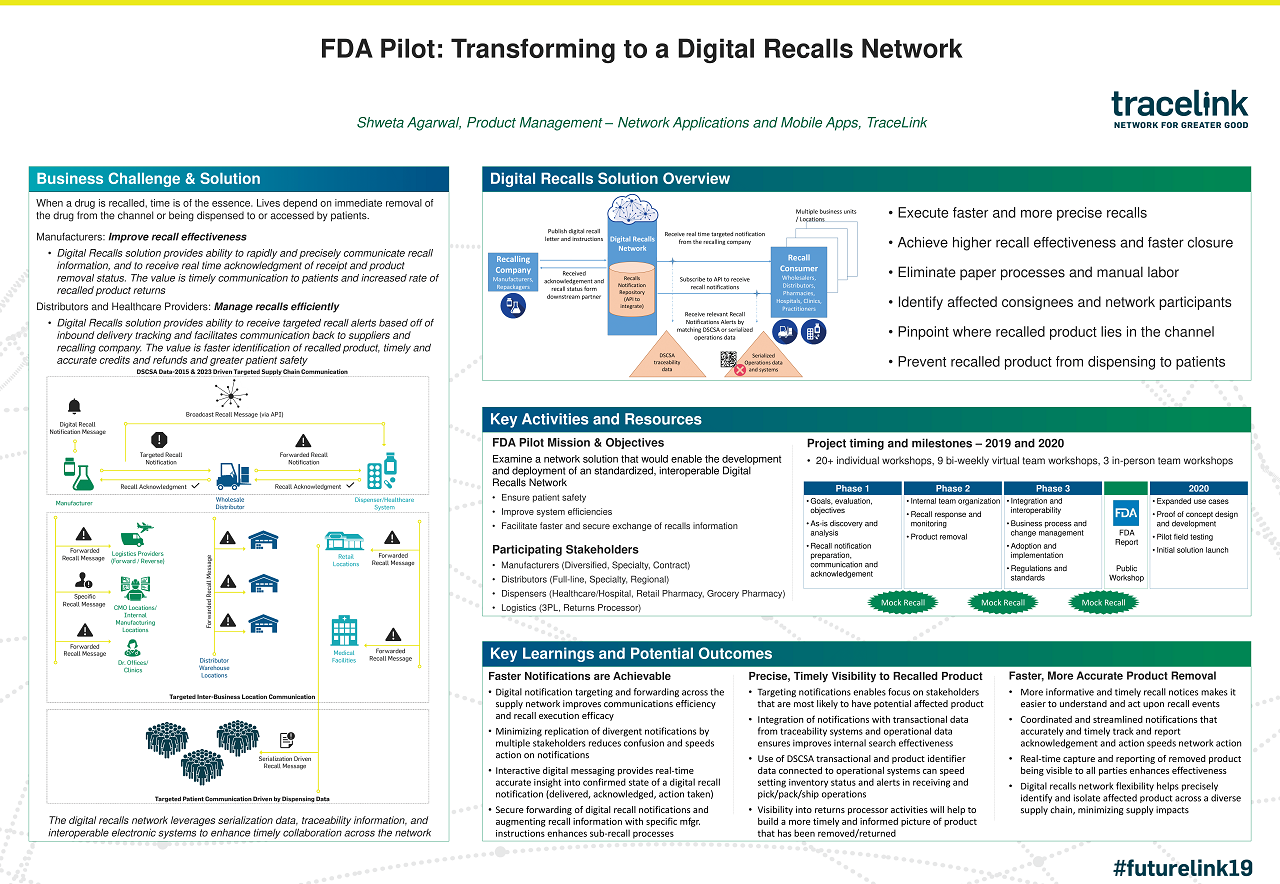
Case Study: TraceLink | FDA Pilot - Transforming to a Digital Recalls Network
Find out how TraceLink helps pharmaceutical manufacturers and dispensers manage recalls more quickly and efficiently than ever.

Case Study: Noden Pharma | The Cost of Non-Compliance
See how global pharmaceuticals company Noden Pharma avoided the financial and operational risks of DSCSA noncompliance.

FDA Announces Enforcement Delay for Manufacturers But Law Still in Effect
Understand what the announcement means for pharma companies as the November 2017 deadline approaches.
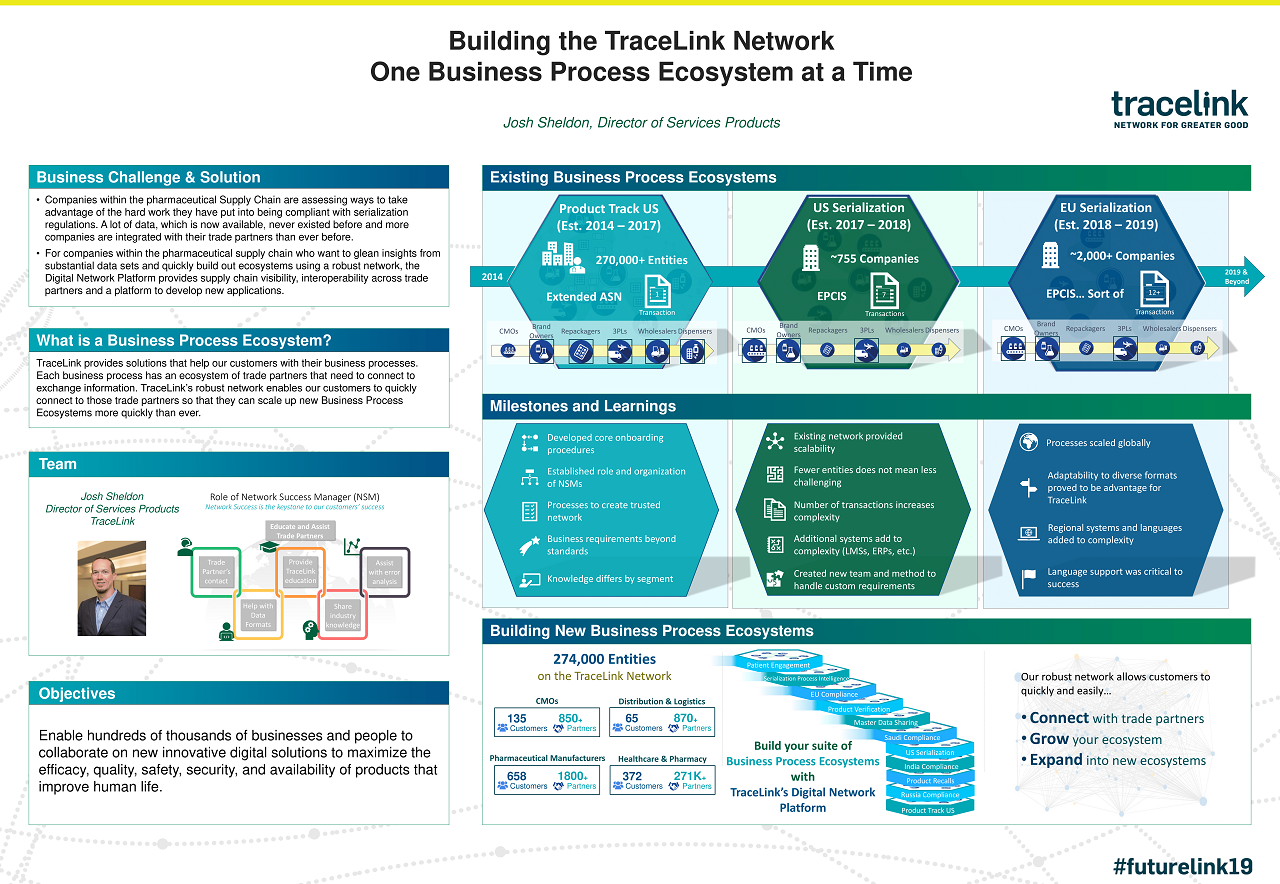
Case Study: TraceLink | Building the TraceLink Network One Business Process Ecosystem at a Time
See how TraceLink's powerful digital supply network enables customers to quickly connect to trade partners for scaling up business process ecosystems.
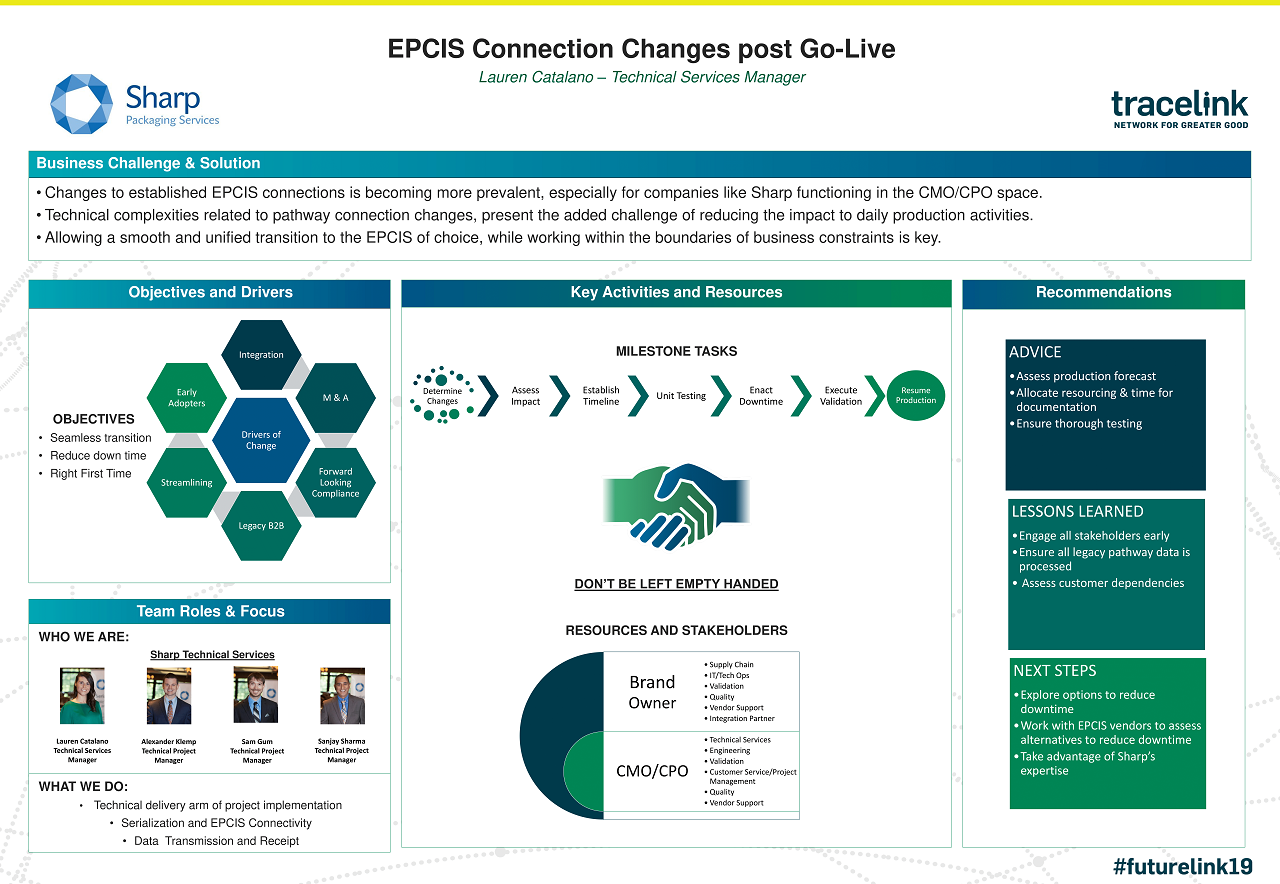
Case Study: Sharp Packaging Services | EPCIS Connection Changes Post Go-Live
See how Sharp Packaging Services overcame EPCIS change management challenges in the pharma supply chain with TraceLink's help.

