United States
Thank you for contacting us; we’ll be in touch shortly.
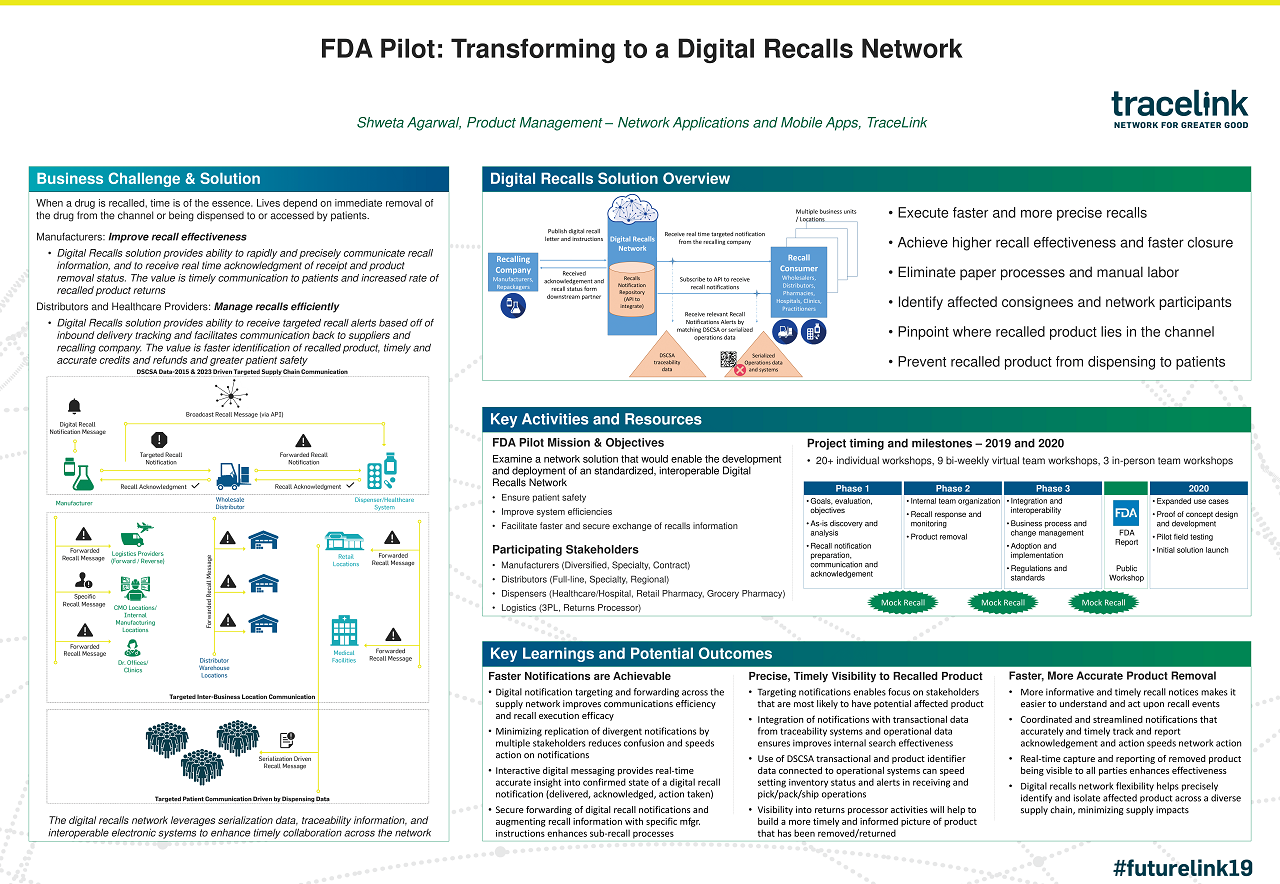
Case Study: TraceLink | FDA Pilot - Transforming to a Digital Recalls Network
Find out how TraceLink helps pharmaceutical manufacturers and dispensers manage recalls more quickly and efficiently than ever.

Case Study: TraceLink | FDA Pilot - Product Traceability Under 2023 DSCSA Regulations - A Business Process-Led Design for a Blockchain Network
TraceLink's breakthrough blockchain solution, Trace Histories, can help pharma customers comply with US DSCSA regulations that go into effect in 2023.
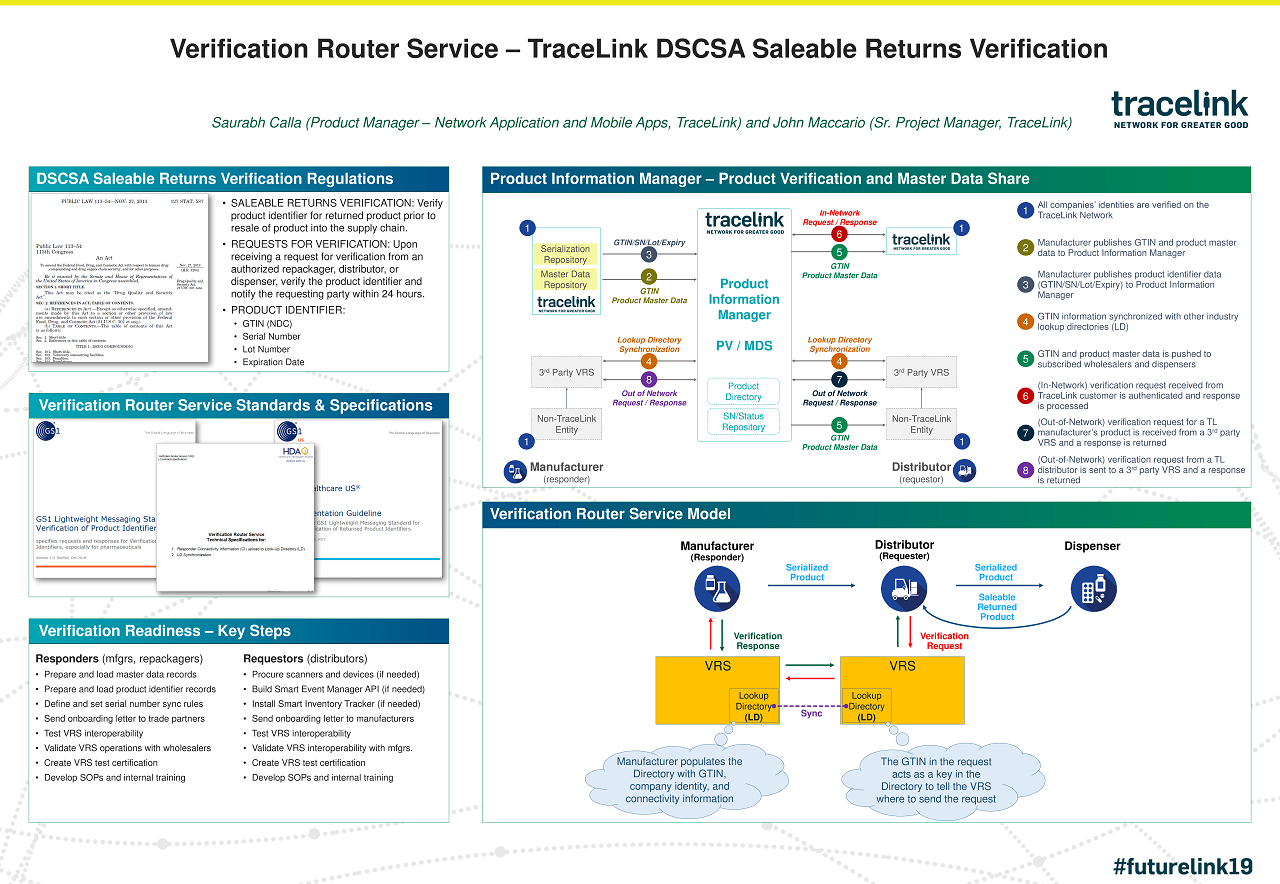
Case Study: TraceLink | Verification Router Service - TraceLink DSCSA Saleable Returns Verification
TraceLink helps customers meet DSCSA saleable returns verification requirements via the Verification Router Service model. See how.
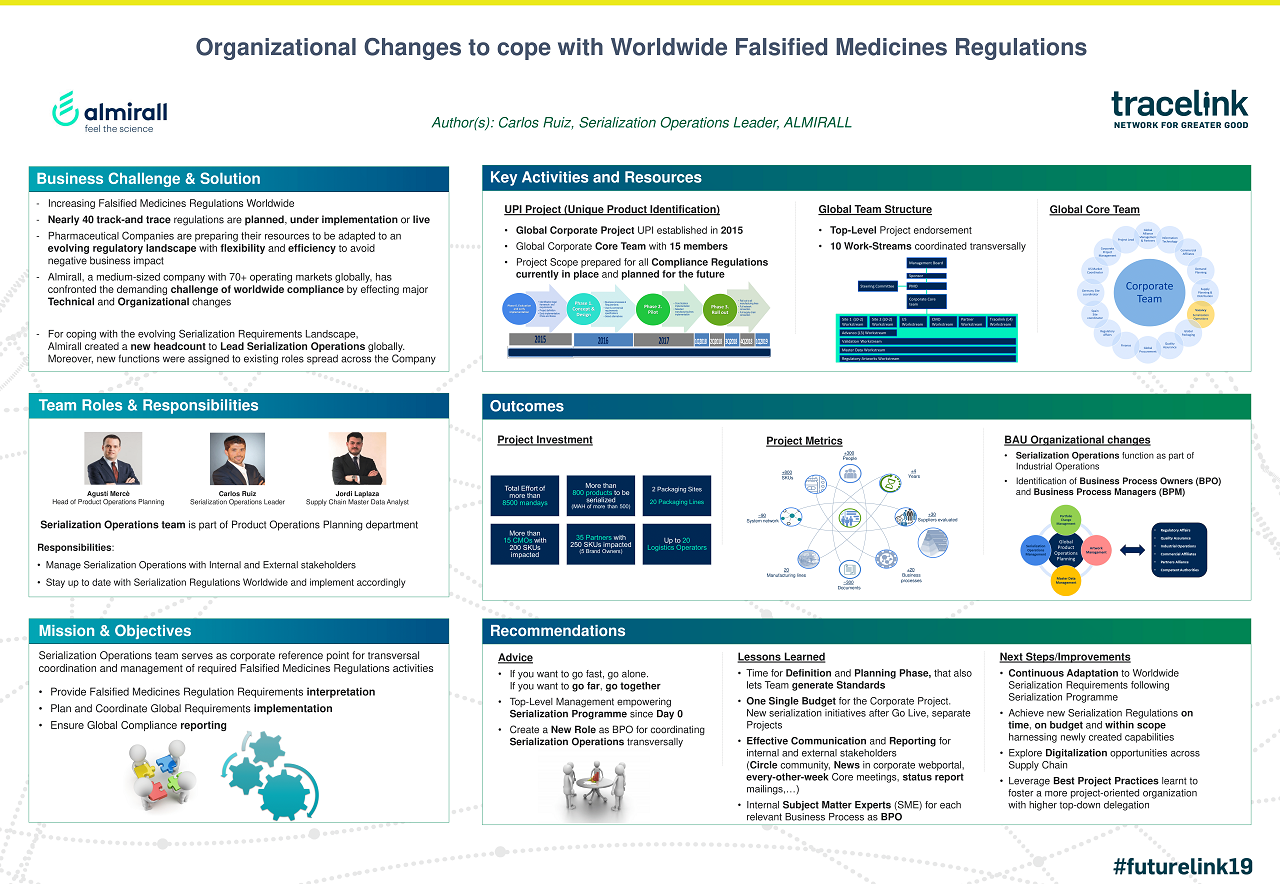
Case Study: Almirall | Organizational Changes to Cope with Worldwide Falsified Medicines Regulations
See how TraceLink helped customers like Almirall implement global compliance strategies to comply with worldwide falsified medicine regulations.

Case Study: TraceLink | Partner with TraceLink to Achieve Compliance and Highlight Your Success
Comply with global track and trace regulations and showcase pharma supply chain compliance. See how TraceLink helps.

Case Study: Value Drug Company | DSCSA Product Investigation—A Compliance Solution
See how Value Drug Company standardized the process for illegitimate and suspect product investigations for DSCSA compliance.

Serialization and Beyond: The Sharp Packaging Story
The mission of U.S. contract packager Sharp Packaging is to drive the convergence between the physical package and the digital data points as they…

FDA Issues Long-Awaited Grandfathering Guidance
Insights into the November 27, 2017, FDA draft guidance around grandfathering product under DSCSA.

FDA Issues Guidance on DSCSA Waivers, Exceptions and Exemptions
In May 2018, the FDA published new DSCSA guidance.

Minimizing Risk: Prepping for Serialization with Pharmas & CMOs
Pharmaceutical and CMO collaborations will be one of the most challenging aspects of serialization projects. Learn how to prepare together.

FDA Announces Enforcement Delay for Manufacturers But Law Still in Effect
Understand what the announcement means for pharma companies as the November 2017 deadline approaches.

Planning for Serialization: A Top-Down Approach
Serialization challenges make it critical to start strategizing from the network down to the device level. Watch this to prepare your IT operations.

