United States
Thank you for contacting us; we’ll be in touch shortly.

Serialization: Where to Begin
Understand the DSCSA serialization requirements, and how they can impact small pharma organizations.
A Make-or-Break Decision: Choosing the Right Serialization Technology for Revenue, Operational, and Brand Success
Understand the serialization challenge in the pharma supply chain and how relational vs. NoSQL databases fare in meeting it. Get the whitepaper now.

The TraceLink Life Sciences Cloud: Talking to Companies in Their Own Format
The TraceLink network plays translator for more than 235,000 companies, seamlessly exchanging compliance data with customers, suppliers and trade…

Checklist: Estimating Your Total Cost of Ownership
Discover what questions to ask when developing a serialization budget to ensure you cover your TCO.

Top 30 Insights from Top Pharma, Sagent, Patheon & McKesson
Continued insights from the speakers at our DSCSA Serialization Workshop: Pfizer, Sagent, Patheon and McKesson.

Planning for Serialization: A Top-Down Approach
Serialization challenges make it critical to start strategizing from the network down to the device level. Watch this to prepare your IT operations.

Understanding the Serialization Challenge: The Actelion Story
Hear Actelion discuss the importance of track and trace, how they approached the serialization challenge, and why they believe you can’t start too…

Ahead of the Curve: Wholesalers, Serialization, and Business Value
Learn more about wholesale distributors who have already begun to explore the potential value of serialized data.

Case Study: PharmaLink | Closing the Gap on Cradle-to-Grave Traceability via Reverse Distribution and EPCIS
Learn how pharma returns specialist PharmaLink increased pharma supply chain security by combining decommissioning and secure product disposal.
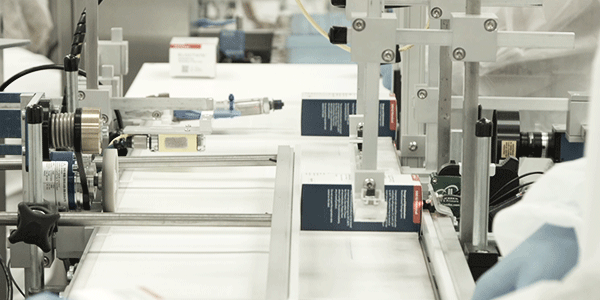
Go-Live: Watching the First Serialized Product Scan for PAR Pharmaceutical
PAR Pharmaceutical captured what a successful serialized workflow looks and feels like, with their first live serialized packaging.

CMOs and CPOs: Best Practices for Approaching Serialization
CMOs and CPOs are critical to the life sciences supply chain’s ability to meet global serialization requirements. Learn how to prepare.

Aggregation: Why is it Such a Hot Topic?
While aggregation isn’t a regulatory requirement in the U.S., understand how the expectations of your wholesale distributor customers may impact your…

