Table of contents
A seismic shift is coming for wholesale distributors and pharmaceutical manufacturers in the form of the Drug Supply Chain Security Act (DSCSA) Saleable Returns Verification requirement.
;
By November 2019, wholesale distributors will be required to verify the serialized product identifiers for any saleable products that have been returned to them before they can be restocked and resold. This requirement introduces enormous challenges for both wholesale distributors and manufacturers, as wholesale distributors will need fast, secure access to manufacturers’ product data in order to minimize operational impact and ensure that saleable products are returned into the supply chain as quickly as possible.
A challenge for wholesalers and manufacturers alike
Saleable returns are estimated to represent approximately 2% of gross sales* for wholesale distributors, based on an annual volume of 59 million returns—nearly a quarter million per day. Manufacturers, who will need to handle hundreds to thousands of verification requests daily, also have a significant stake in reducing the operational impact caused by inefficient, inconsistent saleable returns processes.
Currently, when wholesale distributors exchange information with manufacturers to keep track of incoming product, they often rely on solutions such as point-to-point connections, manufacturer portals, and even paper-based processes. All of these were designed to handle transactions moving in one direction, and do not provide fast bi-directional access to the manufacturer data required to verify a saleable return.
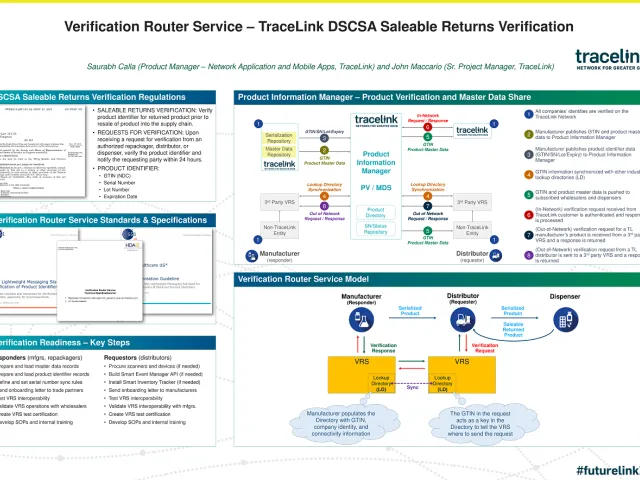
To manage the scope, scale, and challenges of the saleable returns requirement, wholesalers and manufacturers must re-evaluate their current methods of exchanging master data information.
Two approaches to verification
By November 2019, wholesale distributors must be able to verify the serial number, National Drug Code (NDC), lot number, and expiration date of a return against product master data contained in the manufacturer's system of record to ensure that it is a legitimate product that can be resold.
There are two approaches that can be taken for this verification process:
- Self-verify: Receive and store data from the manufacturer when a product is shipped, and refer to that data to verify a saleable return. With aggregation in place, the pallet or case can be scanned to accomplish this. If
product is not aggregated, however, every saleable unit would need to be scanned in order to collect the necessary data. - Verification Router Service (VRS): Use an automated service that looks up the required information in a manufacturer’s product database.
The advantages of Verification Router Services
DSCSA requires that manufacturers respond to requests for saleable return verifications within one business day. However, most wholesale distributors will expect near real-time response to their requests in order to keep warehouse operations running smoothly.
Verification Routing Services play a critical role in allowing wholesalers and manufacturers to meet both the DSCSA requirement and trade partner
- Lookup Directory: Operates as a phone book of GTINS, linking to the responding system where a GTIN can be verified. Lookup Directories are synchronized across VRS systems.
- Request management: Handles a request initiated and submitted by a wholesale distributor to verify the four required data components.
- Response management: Handles a response with the results of the verification.
A significant advantage of a Verification Router Service is its ability to report the current product status. If a product is recalled or withdrawn from the market by the manufacturer after it was shipped, for instance, that information would be available through a verification service but not through self-verifying, where the serial number would still show as valid even though the product was no longer suitable for resale. An automated solution can also furnish an audit trail, retaining a precise record of the query and response and allowing customers to furnish verification evidence quickly in response to a DSCSA request.
What to look for in a VRS
Establishing bi-directional connections between wholesalers and manufacturers will be essential for companies that want to minimize the operational impact of saleable returns verification after November 2019. Important considerations of what to look for in a VRS include:
- Was the Verification Routing Service created based on open interoperable standards?
- Is the VRS accessible by your direct and indirect partners?
- Can the VRS provide sub-second responses to verification requests?
- Are there secure methods to control access to serialization data for verification requests?
- Does the VRS support additional messaging to indicate that a valid serial number has been withdrawn or recalled?
- Does the VRS provide reporting and analytics on the verification data?
- Can the VRS be extended to business use cases such as secure distribution or patient engagement?
Solving verification with Product Information Manager
Automating the saleable returns verification process depends on the ability for partners to manage and share information on a secure, scalable platform. TraceLink’s Product Information Manager delivers master data sharing, verification routing
A network application, Product Information Manager leverages interoperable industry standards and is built on top of TraceLink’s industry-leading digital information-sharing platform.
*HDA Member Survey