Table of contents
On June 21, China’s National Medical Products Administration (NMPA) published a “call for comments” on two new standards for drug traceability that could have a significant impact on how manufacturers prepare their products—and data about those products—for the China market. Key takeaways include:
- NMPA has ”visualized” how data about domestic and imported products would be displayed as a result of a patient query.
- In addition to the EDMC or GS1-standard product identifier, the label itself must be clearly identified as the “Drug Traceability Code” to distinguish it from other barcodes.
- The printing specifications include provisions for both linear barcodes and GS1-compliant 2D data matrix barcodes.
The new standards are in addition to the 10 standards that have already been published since 2019. NMPA is requesting feedback by July 20, 2021.
NMPA Printing Specification of the Drug Traceability Code
The Drug Traceability Code (DTC) is defined in the 2019 standard, “Guidelines for the Construction of the Drug Information Traceability System,” as a unique sequence of numbers, letters and/or symbols that identify each level of a drug’s packaged unit. The June 2021 draft specifications provide additional requirements on the quality and appearance of the DTC:
- Labels must be readable by humans as well as by scanning equipment.
- Labels must have a clear image of the DTC and appropriate contrast with background.
- Labels must include the words “Drug Traceability Code.”
- Labels must be easily locatable on the package.
- Labels must be readable either from left-to-right or top-to-bottom.
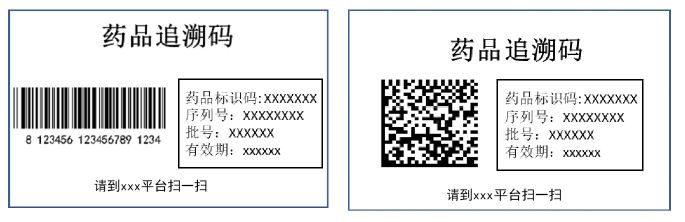
In addition to specifications for linear and data matrix barcodes, the June 21 draft also includes guidance on application and placement of the DTC, including provisions for drug packages fewer than 10 square centimeters; distinct requirements for 1-dimensional linear barcodes and 2-dimensional data matrix barcodes; and the correct application of multiple labels bearing the DTC on large packages.
NMPA Drug Traceability Code Consumer Query Results
This new specification complements the existing standard, “Basic Data Set for Drug Traceability Consumer Inquiry,” and the information a patient would see when requesting a medicine’s traceability information from the NMPA’s Drug Information Traceability System.
This new standard reinforces the significant “data gap” between China’s 2015 regulations and the 2019 regulations currently being implemented by the NMPA. For companies importing drugs into the China market, the data set for consumer queries is extensive and appears to be a mix of master data, product data, and government registration and reporting data. Note that this is an unofficial translation of the original Mandarin document:
- Drug traceability code
- Drug status
- National drug identifier code
- Generic name
- English name of the drug
- Chinese name of the imported drug
- Drug standard code
- Dosage form
- Formulation specifications
- Packing specification
- Packaging conversion ratio
- Drug expiration date
- Drug expiration unit
- Drug approval number
- Drug approval number expiration date
- Registration number of the imported drug
- Expiration date of the registration number of the imported drug
- Imported drug batch number
- Expiration date of the Imported drug batch number
- Drug registration classification
- Drug expiry date deadline
- Batch number
- Drug usage department
- Health agency code
- National standard drug identification
- Special drug management classification
- Prescription drug identification
- Name of the holder of overseas drug marketing authorization (English)
- Unified social credit code (holder of overseas drug marketing authorization)
- Name of the holder of domestic drug marketing authorization (English)
- Unified social credit code (holder of domestic drug marketing authorization)
- Name of overseas drug manufacturer (English)
- Unified social credit code (overseas drug manufacturer)
- Name of repackager
- Unified social credit code (repackager)
- Name of importer
- Unified social credit code (importer)
- Drug manufacturing date
- Unified social credit code of the Drug usage department
- Name of drug retailer
- Unified social credit code (retailer)
While the document provides visualizations for domestically produced and imported medicines and specifies that it is to be free of any adware, it does not describe the process of submitting a query or indicate if a web-based platform or API links might be used to respond to a barcode scan.
Is your company prepared for the new NMPA traceability requirements?
As the NMPA continues to release more detailed specifications on its 3-tier traceability system, companies can expect new challenges as they adapt their China packaging and serialization solutions to meet the new, more complex requirements. TraceLink customers can stay ahead of the latest developments by joining one of TraceLink’s China Special Interest Groups. To register, visit the TraceLink communication preference center:
- Select “Join the TraceLink Community” checkbox to display Community options.
- Select the “China” Community.
- A Mandarin-speaking group is also available: Select “Greater China Region (Mandarin).”
- Scroll to the bottom of the page and select the “Update Communication Preference” box to submit your request.




