Table of contents
The Nov. 27 DSCSA compliance deadline for the final phase of the U.S. Drug Supply Chain Security Act is quickly approaching. With less than five months left to get ready, how has the industry progressed toward meeting compliance requirements? Where have there been successes and where are there roadblocks that need to be addressed ahead of the deadline?
TraceLink recently conducted its own industry assessment to answer these questions. Our benchmarking survey dove deep into a range of preparatory activities and capability deployments from EPCIS data exchange and beyond to assess DSCSA compliance readiness. Polling more than 100 pharmaceutical supply chain companies, including both TraceLink customers and non-customers, we asked pertinent DSCSA questions, such as:
- How far along are you in the process of defining your DSCSA strategy, creating your budget, selecting your technology providers, establishing SOPs, and onboarding partners?
- To what degree will you automate core DSCSA activities between your partners, like EPCIS generation and receipt, master data updates, exception management, and credentialing?
- How ready do you believe you are compared to your peers and your trade partners—and how confident are you that you’ll be ready in time for the Nov. 27 compliance deadline?
It quickly became apparent that the extended pharmaceutical/healthcare supply chain isn't ready yet—in fact, there is still confusion about some of the core requirements of the DSCSA. However, many TraceLink customers are confident they’ll be ready for the Nov. 27 DSCSA compliance deadline in time. This article explores some of the early successes, stumbling blocks, and other key takeaways highlighted by the DSCSA readiness survey.
A network approach delivers quick wins for partner onboarding
With more than 290,000 authenticated and onboarded pharmaceutical supply chain organizations on the TraceLink network, TraceLink customers reported more progress in the area of partner onboarding compared to non-customers. This was especially the case among retail pharmacy respondents—57% of retail dispenser customers are “well underway” compared to 33% of non-customers. The TraceLink network makes it easy to onboard partners en masse, and in many cases, a significant portion of non-customers’ partners may already be on the network.
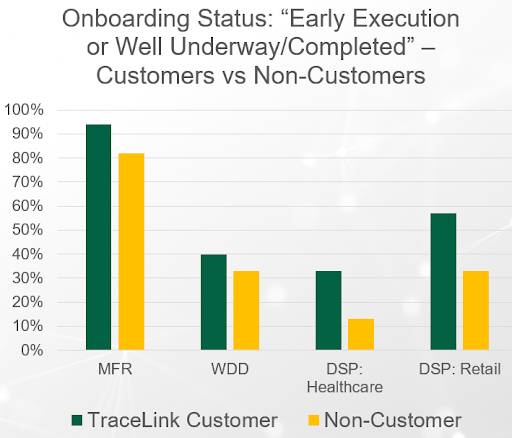
Partner onboarding is one of the five key DSCSA readiness activities that respondents are mobilizing their organization around. Respondents also noted they are "well underway" when it comes to strategy, budgeting, and vendor selection for DSCSA 2023. Comparatively, respondents have made the least progress in authoring new SOPs and updating existing SOPs for the DSCSA 2023 requirements—stakeholders don’t seem to be as focused on post-deadline procedures, like responding to exceptions, doing verifications for suspect products, etc.
Interest in automation is high, but the degree of automation varies
Interoperability is a key requirement of DSCSA 2023 compliance and respondents are not taking a “wait-and-see” approach—they understand the importance of interoperability and aspire to automate multienterprise DSCSA processes. However, the degree to which they intend to automate different processes depends on the respondent’s specific business role in the supply chain.
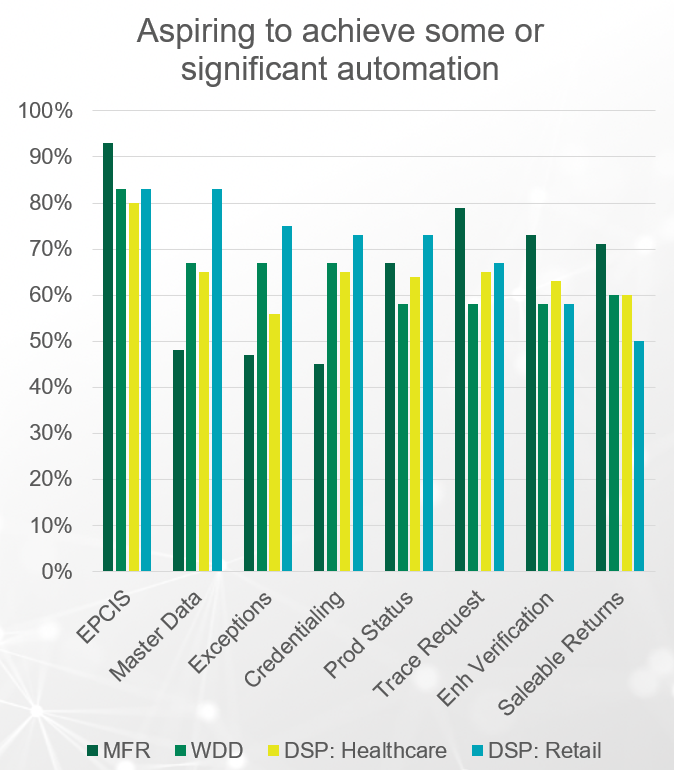
While most (80+%) agree on the need for a high degree of automation in sending and receiving EPCIS data, their views are split by business segment when it comes to how much they intend to automate master data, exception handling, credentialing, and verification. Combined with the fact that as many as 17% of healthcare dispensers and 14% of wholesale distributors were unaware of or did not understand the requirement for automating EPCIS sending and receiving, this highlights there is still confusion around core DSCSA requirements.
The industry is optimistic about DSCSA readiness, but should it be?
Manufacturers, health systems, and retail dispensers are optimistic about their progress toward meeting DSCSA compliance requirements, rating themselves on par or ahead of their peers and trade partners. Wholesale distributors are the outlier, with nearly half rating themselves behind their peers. A similar trend was noted in respondents' confidence in being ready for the compliance deadline on Nov. 27.
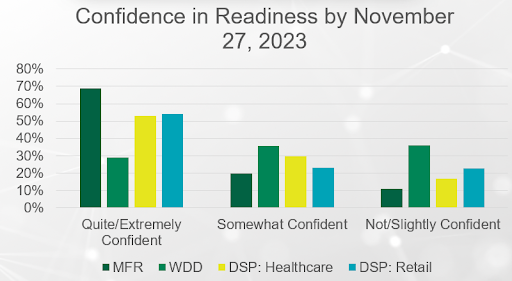
While respondents generally expressed high confidence in their ability to meet DSCSA requirements by the compliance deadline, this sentiment contradicts broader industry signals. For example, in June, the Healthcare Distribution Alliance (HDA) proposed a phased approach for achieving the product traceability and security goals of DSCSA that includes enforcement discretion for wholesale distributors and dispensers. The HDA sent this letter to the FDA due to concerns over whether manufacturers will be able to send accurate package-level data by the compliance deadline.
This also aligns with anecdotal evidence from the TraceLink Services and Network Success teams, as well as input from several TraceLink partners. TraceLink believes this confidence indicates a lack of understanding of the preparation that needs to be done, especially for non-TraceLink customers. For all TraceLink customers that have not yet sent EPCIS test data, we recommend that you contact TraceLink to have your account executive setup a meeting to review your DSCSA readiness. TraceLink can get most customers ready in a few hours to a few days, but we need to talk with you first.
For a detailed look at the benchmarking survey and the results, watch the webinar—you may be able to glean some insight to guide your own DSCSA readiness initiatives. You’ll also get a copy of the presentation slides when you sign up for the webinar recording.





