Table of contents
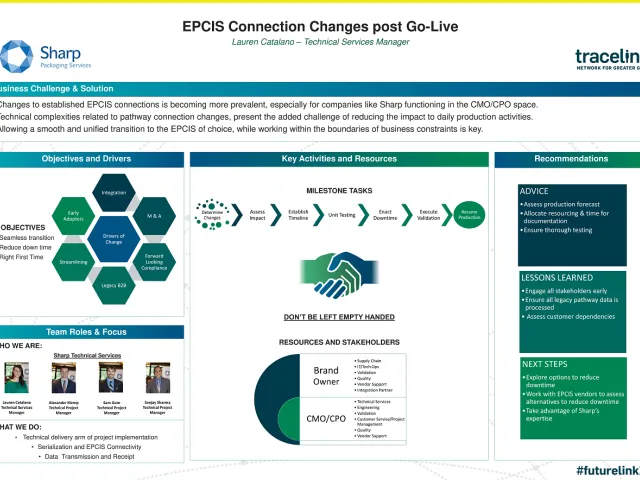
If you do business in the U.S. and EU, your approach to DSCSA and FMD compliance will not be the same. Hear Sharp technical executives discuss the similarities and the critical differences between the two regulations to help you prepare.