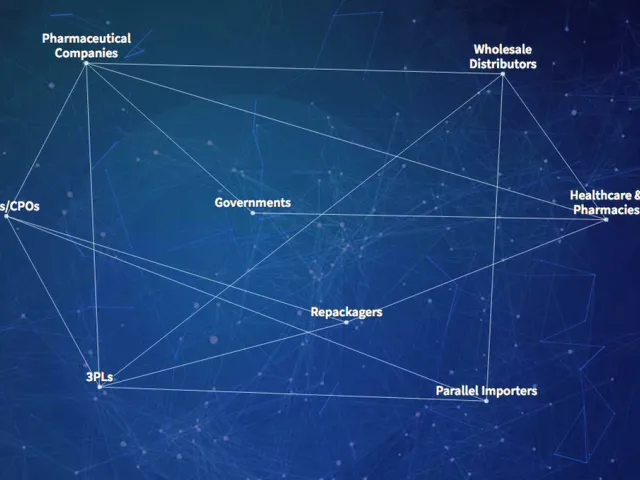
Table of contents
See how CMO Medreich PLC developed a compliant label for its EU FMD-compliant anti-tampering devices for prescription medicine packs, all while protecting the efficiency of their line systems. Medreich’s poster, “Medreich PLC Designing and Using of Anti-Tampering Device,” was one of 11 featured during FutureLink Barcelona’s interactive Poster Sessions.