Table of contents
Read the transcription
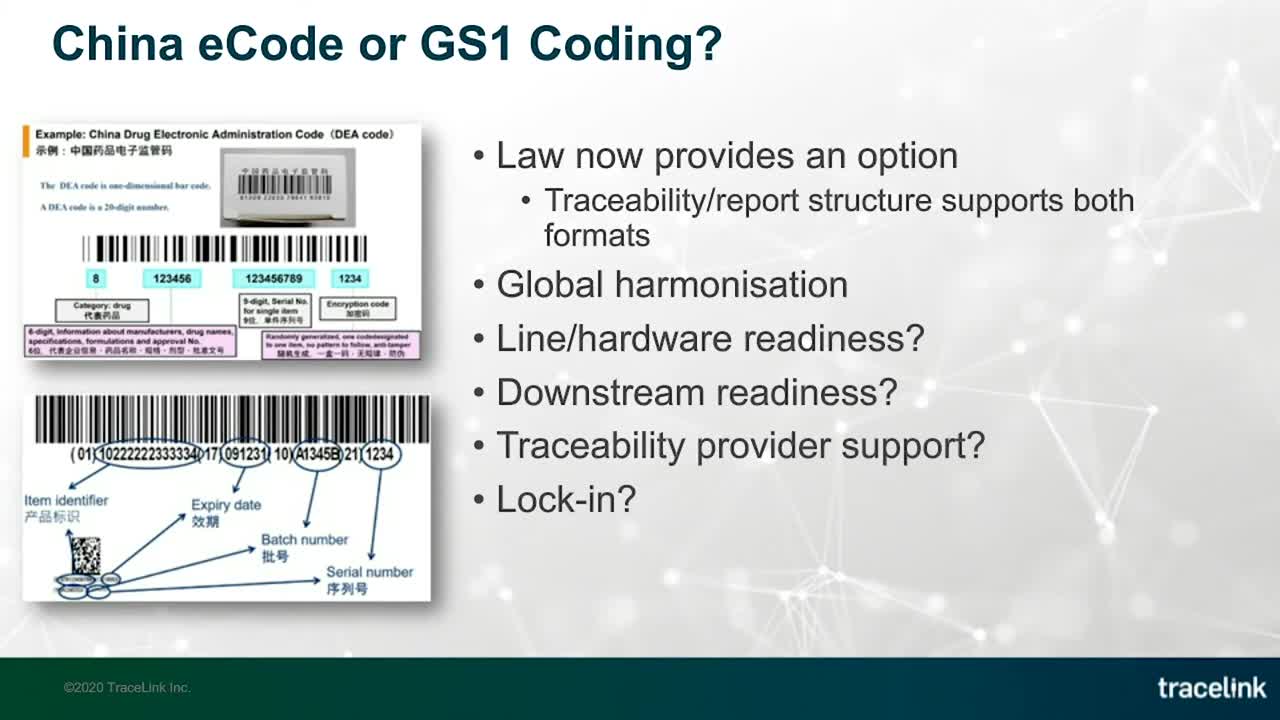
Since 2015, China’s track and trace regulations have specified that products be identified using the 20-character Chinese eCode standard. In 2019, the guidelines were amended to include a second encoding option that conforms to the GS1 coding standard used by manufacturers to meet traceability regulations in markets around the world.
Which one is right for you? Watch this short excerpt from our recent webinar, China's Technical Requirements and Compliance Deadlines: What You Need to Know Now, to learn more and to understand key considerations for making the right choices.





