-
Resource
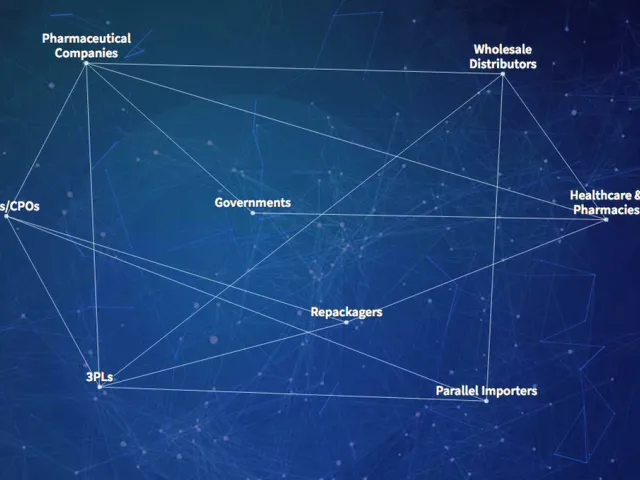
Saleable Returns Briefing: Who is Affected?
Get the latest on the DSCSA saleable returns verification requirements and deadlines in 12 minutes.
View More
-
Resource
Accelerate Serialized Operations with Serialized Product Intelligence
Serialized Product Intelligence lets you proactively search, filter, and analyze serialization data and drill down to quickly pinpoint potential problems.
View More
-
Resource
TraceLink University: Mastering Serialization with Continuous Learning
Learn how TraceLink University can support your education and compliance needs.
View More
-
Resource
Mutual Drug Q&A: Understanding Saleable Returns and Verification
Wholesale distributor Mutual Drug is getting ready for the DSCSA saleable returns verification requirement. Read how in this Q&A.
View More
-
Resource
Poll: Fast Data Exchange, Security Are Top Saleable Returns Priorities
Polls show that when choosing a VRS, manufacturers and wholesalers consider network governance, EPCIS and master data exchange, and interoperability.
View More
-
Resource
Getting Ready for DSCSA Serialization: 5 Considerations for Wholesale Distributors
Get ready for DSCSA serialization. Find out how wholesale distributors will be impacted by 2019's DSCSA serialization requirement.
View More
-
Resource
Industry Pulse: Warehouses Not Ready for EU FMD
Learn about EU FMD warehouse-related requirements for compliance.
View More
-
Resource
EU FMD and Risk-Based Verification in the Warehouse: Top Questions Answered
Learn the answers to six verification questions surrounding EU FMD and warehouses.
View More
-
Resource
Henry Schein: Manufacturers Must Stay Focused on Saleable Returns Verification
Wholesaler Henry Schein executives offer insights on why suppliers need to implement a VRS to ensure warehouse efficiency and customer satisfaction.
View More
-
Resource
160+ TraceLink VRS Customers Ahead of Pace for Saleable Returns Deadline
Learn why TraceLink continues to set the pace in VRS readiness, live customers, and industry-wide interoperability.
View More
-
Resource
DSCSA Verification: VRS Essentials for Manufacturers and Wholesalers
Before selecting a Verification Router Service to meet 2019 DSCSA requirements, see what wholesales and manufacturers need to ask.
View More
-
Resource
Serialized Product Intelligence Creates a Foundation for Game-Changing Analytics
Learn how TraceLink helps companies resolve supply disruptions and build a foundation for next-level analytics.
View More
-
Resource
Is Your ERP System Really “Serialization-Ready?”
Before you turn to your ERP to handle complex DSCSA compliance and serialization operations, ask these important questions.
View More
-
Resource
Preparing for DSCSA Saleable Returns Verification
Understand the impact of the upcoming DSCSA saleable returns verification requirement for both pharma companies and wholesale distributors.
View More
-
Resource
5 Risk Factors of Using Your LMS Provider to Meet Brazil Compliance Requirements
Learn why Line Management System (LMS) vendors can’t compare to TraceLink’s enterprise-level data management capabilities for Level 4 - Level 5 serialization.
View More
-
Resource
Your ANVISA Implementation Plan: Quick Start Guide
ANVISA may require pharmaceutical companies to submit a serialization implementation plan for Brazil as early as December 31, 2020 via the SNCM portal.
View More
-
Resource
Russia Track and Trace Law Signed
Understand how Russia's amended implementation dates and drug class specifications impact your company.
View More
-
Resource
Russia Compliance: Key Regulatory Terms
Learn more about the new and unfamiliar terms that are key to understanding how to comply with Russian regulations.
View More
-
Resource
Cut Through the Complexity with TraceLink’s Russia Compliance Solution
Pharmaceutical manufacturers need to comply with the Russia's highly complex track and trace law. See how TraceLink can help.
View More
-
Resource
Brazil Serialization Requirements: Getting Started
Download the visual guide to Brazil's serialization requirements. Get a quick understanding of Brazil track and trace regulations.
View More
-
Resource
China Compliance: Systems and Standards at a Glance
This infographic provides an overview of the key requirements of China’s track and trace law so you can begin your serialization and compliance planning now
View More
-
Resource
A Day in the Life of Handling Recalls
View this infographic to learn the true cost of recall management and how serialization is the right way to handle pharmaceutical recalls.
View More
-
Resource
EU FMD Post-Launch: Your Guide to Compliance, Risk, and Business Value
Read this eBook to learn about the post-launch EU FMD landscape, from regulatory updates to lessons learned to critical components needed for a successful solution.
View More
-
Resource
The Pharmacy of the Future: Embracing the Digital Transformation
How can you get new business value from your investment in serialization? Start here.
View More
-
Resource
FDA Pilot Report: Digital Recalls Network and DSCSA 2023 Traceability with Blockchain
See the results of TraceLink’s FDA pilot project that focused on two workstreams; digital recalls and blockchain with participants from 22 companies across the supply chain
View More
-
Resource
Saudi Arabia: Understanding the Compliance Requirements
Download this infographic for an overview of Saudi reporting requirements, key deadlines, roles and responsibilities, packaging elements, and more.
View More
-
Resource
What You Must Know to Survive a Supply Chain Crisis: 5 Priorities from 4 Industry Experts
Read important insights on building agile supply chains and managing supply chain disruptions, from leading industry and academic thought leaders.
View More
-
Resource
Mutual Drug Discusses DSCSA Compliance Challenges
Hear Mutual Drug discuss their criteria for selecting a DSCSA solution partner plus compliance challenges.
View More
-
Resource
The NATCO Story: Securing Drug Supply through Serialization
India-based NATCO Pharma talks about DSCSA compliance, serialization, and choosing a solution.
View More
-
Resource
Smarter CMO Onboarding: Speed Up Serialization with Network Efficiency
Learn how a network approach is the best way to boost the efficiencies of CMO onboarding with brand owner customers.
View More