Global Track & Trace
Thank you for contacting us; we’ll be in touch shortly.

Master Data and Serialization: Understanding the Bidirectional Impact
Learn about the impact of master data, how it ties into serial numbers, and understand why it's critical to your serialization strategy.
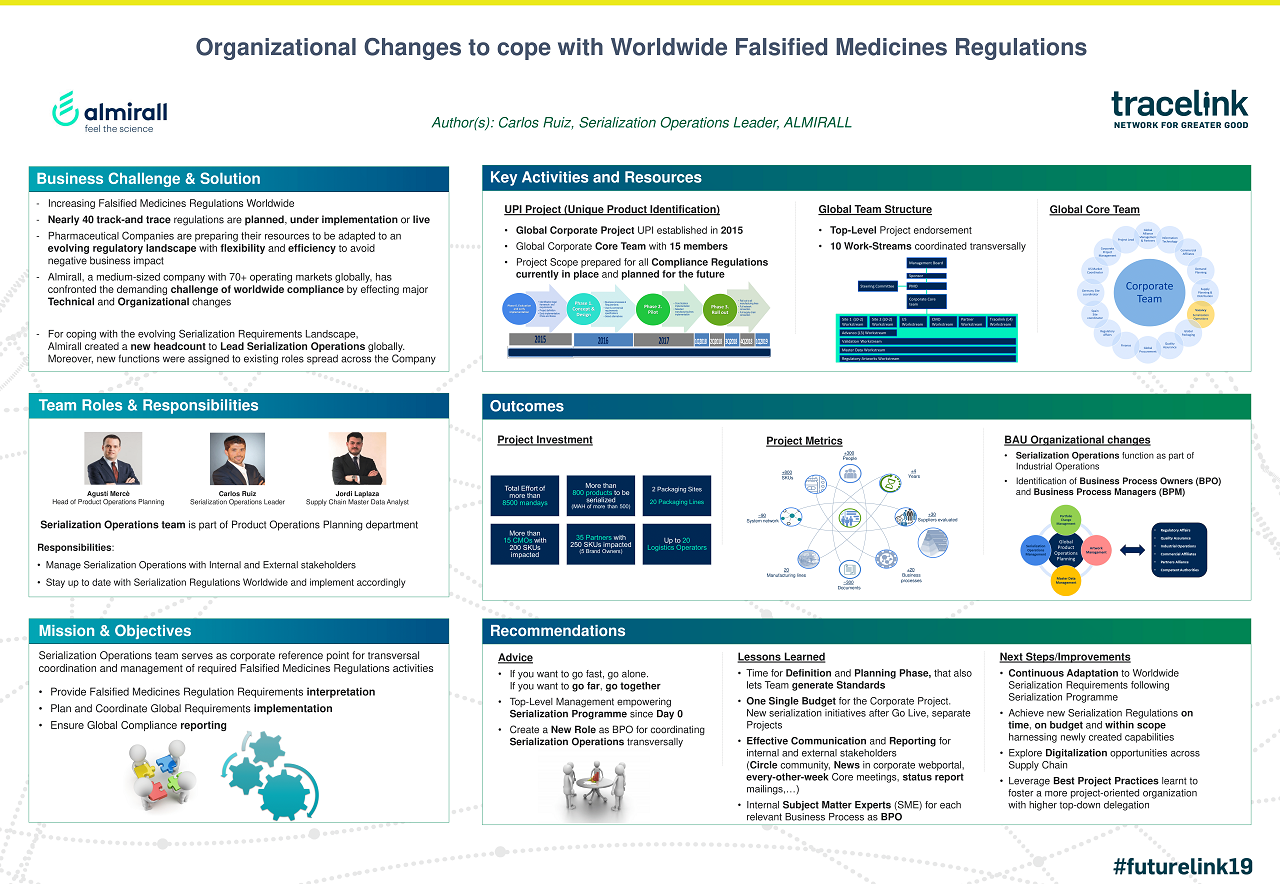
Case Study: Almirall | Organizational Changes to Cope with Worldwide Falsified Medicines Regulations
See how TraceLink helped customers like Almirall implement global compliance strategies to comply with worldwide falsified medicine regulations.

Case Study: TraceLink | Partner with TraceLink to Achieve Compliance and Highlight Your Success
Comply with global track and trace regulations and showcase pharma supply chain compliance. See how TraceLink helps.

Minimizing Risk: Prepping for Serialization with Pharmas & CMOs
Pharmaceutical and CMO collaborations will be one of the most challenging aspects of serialization projects. Learn how to prepare together.
ACS Q&A: Building a National System for EU FMD
Read an interview with ACS PharmaProtect to learn how the German national system works and how it will operate under EU FMD.

Laetus Q&A: How People, Process, and Planning Will Drive Your EU FMD Compliance Success
Track and Trace Manager at Laetus explains why humans have a major impact on traceability, and what insights supply chain companies can gain from…

How EU FMD Impacts CMOs
Find out in under two minutes what CMOs need to do to maintain and win business under EU FMD.

Hidden Costs of Serialization
Are you prepared to navigate the entire serialization iceberg? View a breakdown of all costs you will incur.

UNITAX Ramps Up Business Growth and Meets Compliance Deadlines Ahead of Time with TraceLink
Learn why UNITAX partnered with TraceLink to help them meet the February 2019 EU FMD serialization deadline ahead of schedule.
Altran Q&A: Spain’s Small Pharmas Face Big Serialization Challenges for EU FMD
Understand the challenges small pharmas face as serialization approaches, and how industry expert Altran helps solve them.

What's So Challenging about Continuous Compliance?
In the new regulatory landscape, maintaining compliance is a real challenge.

Serialization Terms for Hospitals and Pharmacies
Learn over 100 serialization terms and what they mean to help you with DSCSA compliance.

