Regulatory/Compliance
Thank you for contacting us; we’ll be in touch shortly.

Case Study: TraceLink | Partner with TraceLink to Achieve Compliance and Highlight Your Success
Comply with global track and trace regulations and showcase pharma supply chain compliance. See how TraceLink helps.

Case Study: Ferrer | Building a Master Data Strategy for EU FMD
Learn how Ferrer worked with TraceLink to manage its master data for EU FMD compliance.

Case Study: IBSA | Using Serialization to Ensure Product Integrity
Learn how IBSA used serialization to protect their product from counterfeiting.

Case Study: Medreich | EU FMD from Project Plan to Post Implementation
See how Medreich and TraceLink collaborated to achieve EU FMD compliance.

Case Study: Medreich | Anti-Tampering and EU FMD
Learn how Medreich designed an EU FMD-compliant label to work with anti-tampering devices.

Case Study: Mithra | Serializing Across Multiple Business Cases
Learn how Mithra used a multidisciplinary approach for a successful EU FMD go-live.
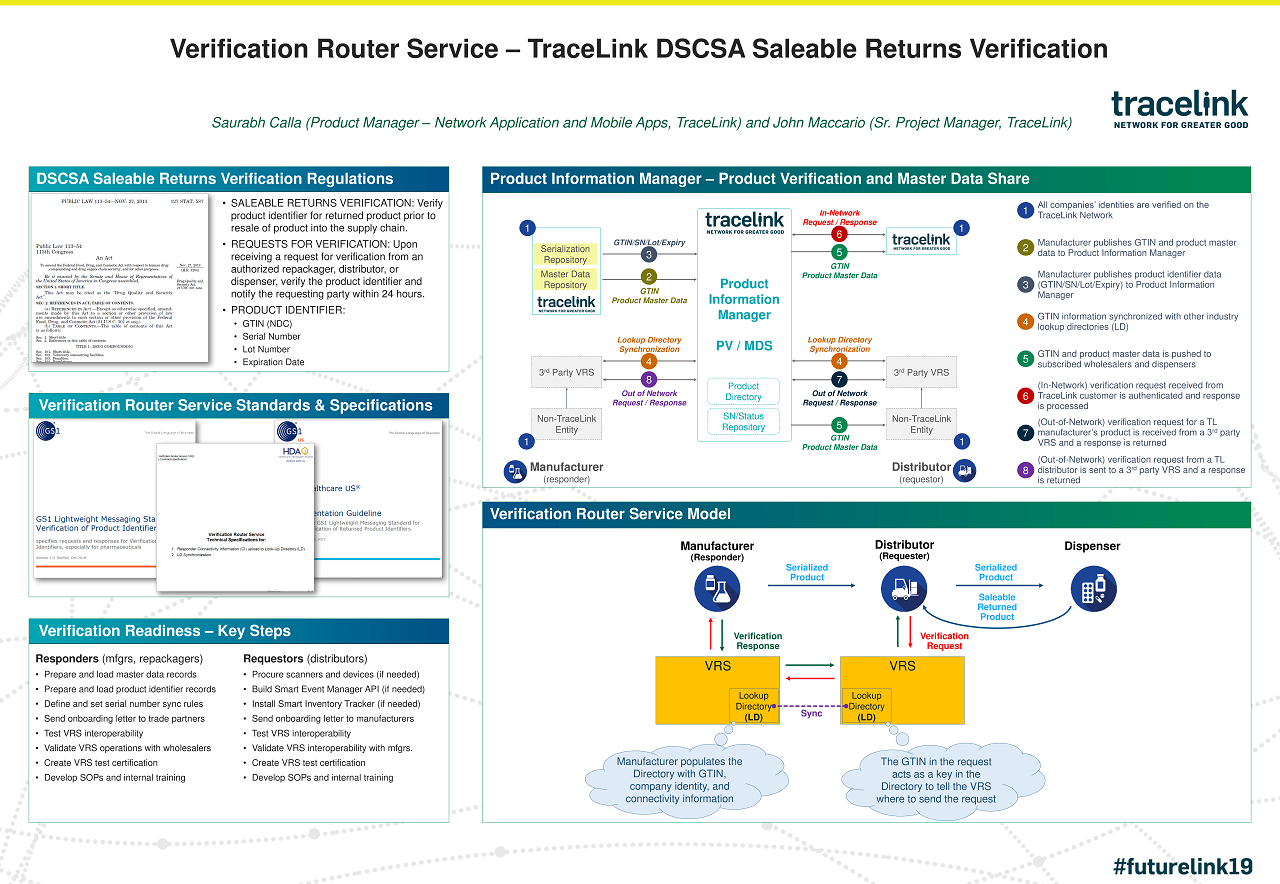
Case Study: TraceLink | Verification Router Service - TraceLink DSCSA Saleable Returns Verification
TraceLink helps customers meet DSCSA saleable returns verification requirements via the Verification Router Service model. See how.

Building for EU FMD - 5 Serialization Leaders Share How to Prepare
See how experts have overcome roadblocks as they implement Level 1-5 solutions for EU FMD serialization.
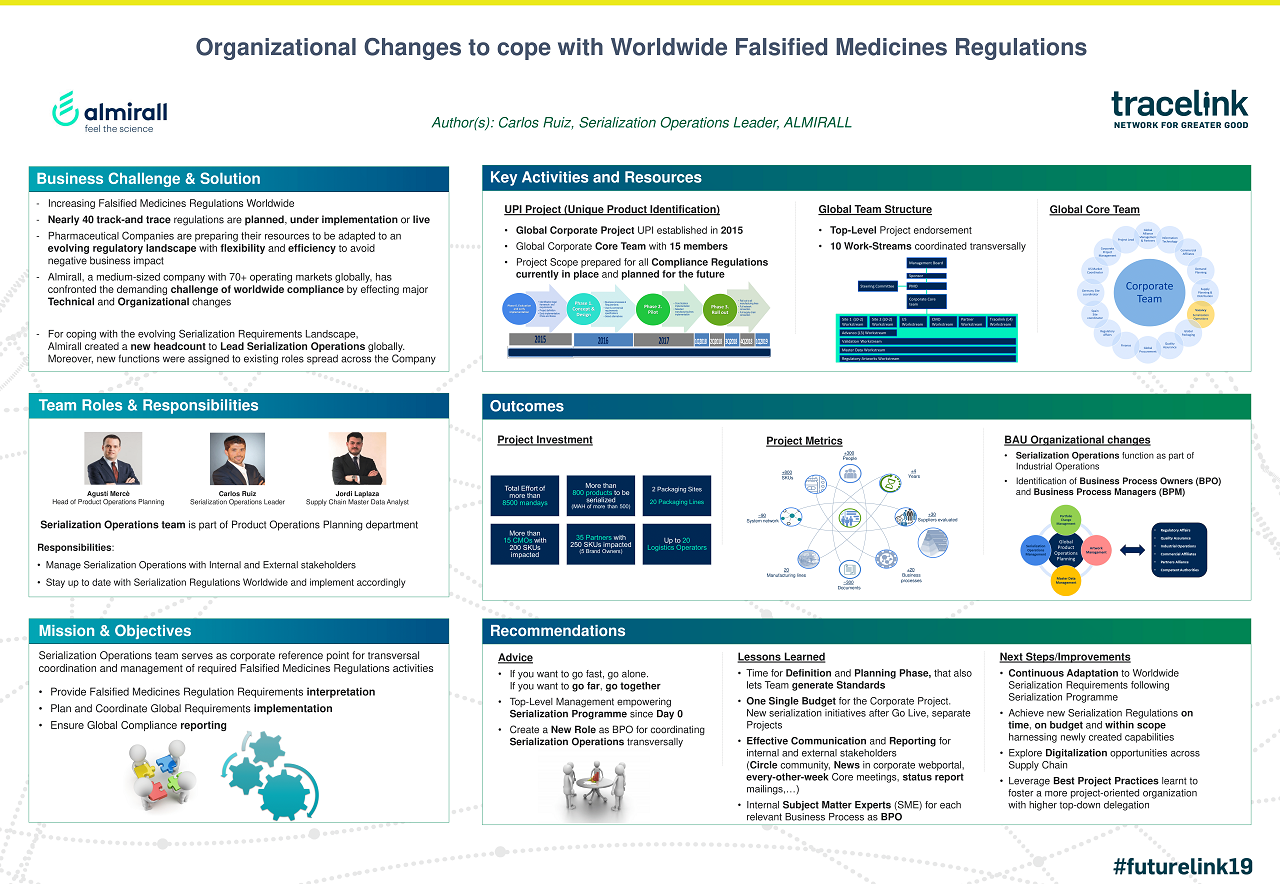
Case Study: Almirall | Organizational Changes to Cope with Worldwide Falsified Medicines Regulations
See how TraceLink helped customers like Almirall implement global compliance strategies to comply with worldwide falsified medicine regulations.

Case Study: Noden Pharma | The Cost of Non-Compliance
See how global pharmaceuticals company Noden Pharma avoided the financial and operational risks of DSCSA noncompliance.

Case Study: TraceLink | FDA Pilot - Product Traceability Under 2023 DSCSA Regulations - A Business Process-Led Design for a Blockchain Network
TraceLink's breakthrough blockchain solution, Trace Histories, can help pharma customers comply with US DSCSA regulations that go into effect in 2023.
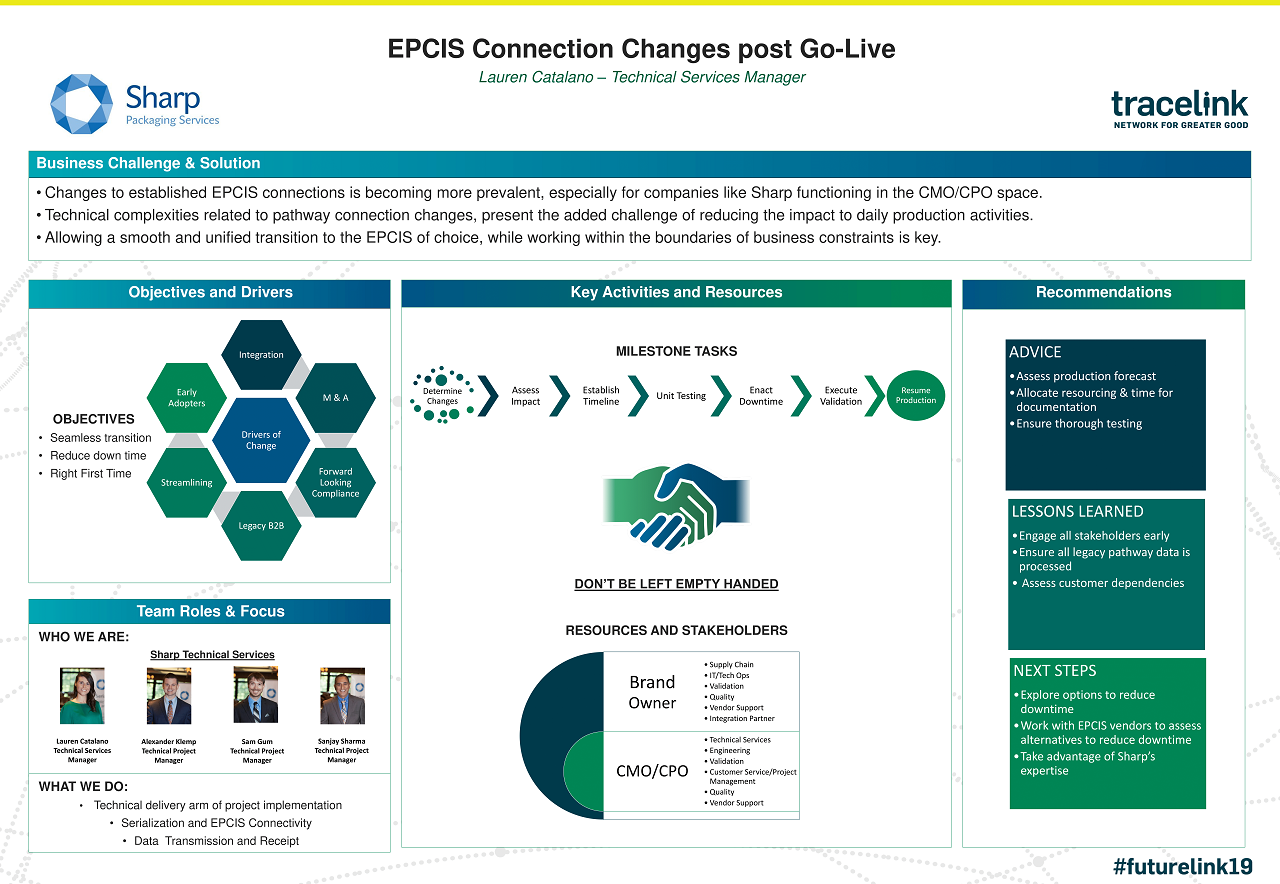
Case Study: Sharp Packaging Services | EPCIS Connection Changes Post Go-Live
See how Sharp Packaging Services overcame EPCIS change management challenges in the pharma supply chain with TraceLink's help.

