Table of contents
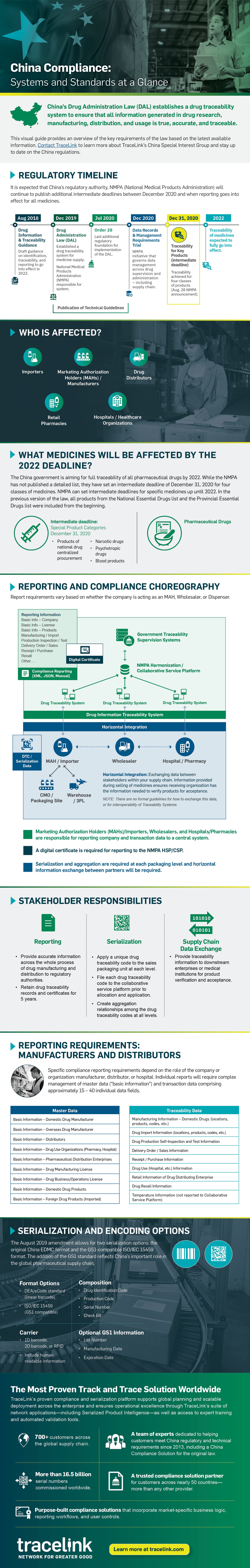
China is quickly ramping up its regulatory infrastructure and setting intermediate deadlines—and you need to be ready. This visual guide provides an overview of the key features of China’s Drug Administration Law so you can begin your 2021 serialization and compliance planning now.
Topics include:
- The latest regulatory timeline, including the December deadline for 4 medicine classes
- China’s 3-tier reporting system and the importance of “horizontal integration” with downstream partners
- Master data and traceability data requirements
- Key differences between China EDMC and GS1-compatible identifiers