Table of contents
Evolving serialization and track and trace requirements necessitate frequent software updates to ensure continuous compliance, and those frequent updates demand ongoing revalidation of systems. For manufacturers struggling to keep up, what are the staff resourcing and budgetary impacts?
In a series of polling questions, more than 50 pharma companies and CMOs shared their experiences.
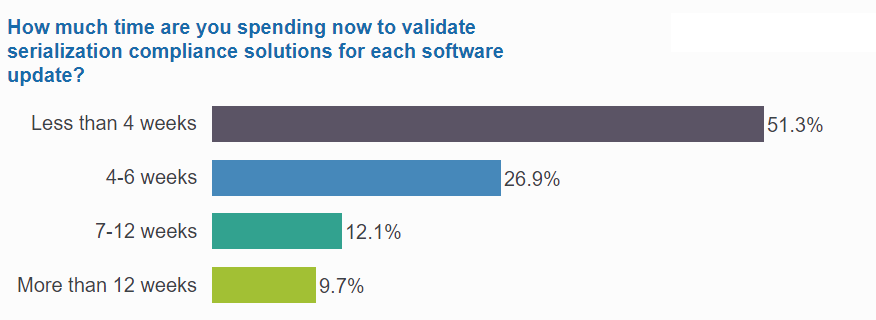
Nearly half spend at least 4 weeks on validation for every software update
Time spent on validating solutions varies depending on the volume of inventory and the size of supply chain networks. Poll respondents split nearly evenly between companies that require less than a month per release, and those that need significantly more time for testing.
For nearly a quarter of the community, validation requires much more than 4 weeks. 12% need at least 7 weeks, while 10% are spending at least 3 months on their validation processes.
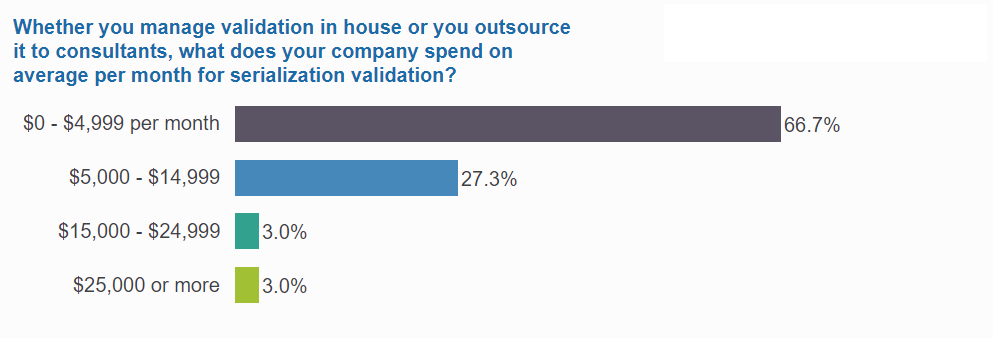
1 in 3 spend over $60K annually on validation
One-third of respondents spend more than $60K annually, and for some, the investment goes much higher: 1 in 5 in this group have devoted at least $180K annually to their validation efforts.
Not only is skilled labor to perform quality testing expensive, it’s hard to find. To overcome the challenge of hiring and training experts, many companies rely on external help, with 2 of 5 outsourcing some or all of the IQ, OQ, and PQ. An additional 22% still haven’t defined their validation strategy, which means that third-party resources may soon become even harder to secure.
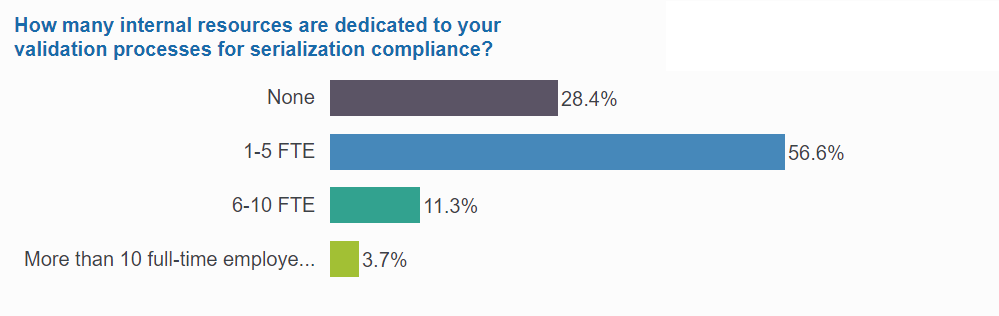
3 of 4 manage validation internally—and often use 6 or more FTE
Validation is tying up internal resources across many organizations, with three-fourths of respondents dedicating internal staff to their validation processes. Among them, 20% are assigning 6 or more full-time employees entirely to validation. And of those, 1 in 5 companies are actually dedicating more than 10 full-time employees to validation.
Automate your entire validation life cycle for serialization
What would you do with the extra time and resources you’d have if you could offload all the validation of your serialization solution through automation? Whether you’re a large enterprise pharma with a team of experts or a virtual brand with no strategy in place, automation means you’ll no longer need to reconfigure internal teams, bring on additional skilled experts, or devote weeks or months of cost and effort to validation with each release.
Automated Validation Manager (AVM) is a new solution that goes beyond the IQ/OQ we already provide by managing the entire TraceLink validation life cycle. Watch how it works in this 20-minute webinar—available on demand.
See how to:
- Completely automate PQ for every TraceLink release.
- Access all tests and save weeks of time and expense.
- Get every release certificate in real time.