
-
Resource
Is Your ERP System Really “Serialization-Ready?”
Before you turn to your ERP to handle complex DSCSA compliance and serialization operations, ask these important questions.
View More
-
Resource
Preparing for DSCSA Saleable Returns Verification
Understand the impact of the upcoming DSCSA saleable returns verification requirement for both pharma companies and wholesale distributors.
View More
-
Resource
5 Risk Factors of Using Your LMS Provider to Meet Brazil Compliance Requirements
Learn why Line Management System (LMS) vendors can’t compare to TraceLink’s enterprise-level data management capabilities for Level 4 - Level 5 serialization.
View More
-
Resource
Your ANVISA Implementation Plan: Quick Start Guide
ANVISA may require pharmaceutical companies to submit a serialization implementation plan for Brazil as early as December 31, 2020 via the SNCM portal.
View More
-
Resource
Russia Track and Trace Law Signed
Understand how Russia's amended implementation dates and drug class specifications impact your company.
View More
-
Resource
Russia Compliance: Key Regulatory Terms
Learn more about the new and unfamiliar terms that are key to understanding how to comply with Russian regulations.
View More
-
Resource
Cut Through the Complexity with TraceLink’s Russia Compliance Solution
Pharmaceutical manufacturers need to comply with the Russia's highly complex track and trace law. See how TraceLink can help.
View More
-
Resource
Brazil Serialization Requirements: Getting Started
Download the visual guide to Brazil's serialization requirements. Get a quick understanding of Brazil track and trace regulations.
View More
-
Resource
China Compliance: Systems and Standards at a Glance
This infographic provides an overview of the key requirements of China’s track and trace law so you can begin your serialization and compliance planning now
View More
-
Resource
A Day in the Life of Handling Recalls
View this infographic to learn the true cost of recall management and how serialization is the right way to handle pharmaceutical recalls.
View More
-
Resource
EU FMD Post-Launch: Your Guide to Compliance, Risk, and Business Value
Read this eBook to learn about the post-launch EU FMD landscape, from regulatory updates to lessons learned to critical components needed for a successful solution.
View More
-
Resource
The Pharmacy of the Future: Embracing the Digital Transformation
How can you get new business value from your investment in serialization? Start here.
View More
-
Resource
FDA Pilot Report: Digital Recalls Network and DSCSA 2023 Traceability with Blockchain
See the results of TraceLink’s FDA pilot project that focused on two workstreams; digital recalls and blockchain with participants from 22 companies across the supply chain
View More
-
Resource
Saudi Arabia: Understanding the Compliance Requirements
Download this infographic for an overview of Saudi reporting requirements, key deadlines, roles and responsibilities, packaging elements, and more.
View More
-
Resource
What You Must Know to Survive a Supply Chain Crisis: 5 Priorities from 4 Industry Experts
Read important insights on building agile supply chains and managing supply chain disruptions, from leading industry and academic thought leaders.
View More
-
Resource
Worldwide Regulatory Updates
Get insights into the most recent worldwide track and trace regulatory compliance updates for the healthcare supply chain.
View More
-
Resource
Introducing Serialized Product Intelligence: Santen Pharmaceutical Shares Its Journey to Supply Chain Transformation
Learn about Santen's journey from serialization to digitalization and the critical role that Serialized Product Intelligence will play in its future.
View More
-
Resource
How Does Serialized Product Intelligence Enable Root Cause Analysis of Compliance Errors?
Watch this product demo to see how Serialized Product Intelligence empowers serialized operations teams with self-service troubleshooting capabilities.
View More
-
Resource
Poll: Russia Crypto Codes Create Unique Operational Challenges Manufacturers Must Address Now
Companies are moving forward with Russia compliance and crypto code strategies. Are you behind?
View More
-
Resource
Rising to the Challenge of Russia Compliance: An Interview with Santen Pharmaceutical’s Pasi Kemppainen
Learn how Santen Pharmaceutical is navigating Russia's highly complex and demanding serialization and track and trace regulations.
View More
-
Resource
Brazil Compliance: A Step-by-Step Approach to Serialization
Begin your Brazil serialization journey and see why you need to start today to meet the April 2022 ANVISA deadline. Download the infographic.
View More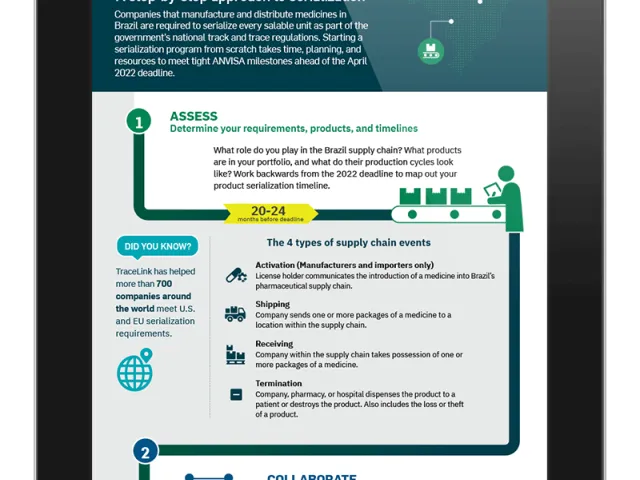
-
Resource
94% Recommend Product Track with End-to-End Administration Services
See how pharmacies continue to realize significant time and cost savings with TraceLink service.
View More
-
Resource
AVM Helps Virtual Manufacturer Automate Software Validation Across Diverse Supply Chain
Read how a leading biopharmaceutical manufacturer turned to TraceLink’s Automated Validation Manager to help manage software compliance.
View More
-
Resource
Why An Open, Standards-Based Approach is Essential for the Pharmaceutical Supply Chain
Learn five critical criteria to determine whether you want a trusted partner or just a vendor.
View More
-
Resource
APD: Partnering to Achieve Data-Driven Outcomes
American Pharmaceutical Distributors is using the TraceLink data platform to improve operational efficiency and customer service.
View More
-
Resource
Aurobindo Pharma, Serialization, and Best of Breed Expertise
Listen as Aurobindo Pharma's CIO explains the impact of serialization, and partnering with best in breed expertise.
View More
-
Resource
Achieving Continuous Compliance with Automated Validation Manager
Learn how TraceLink's Automated Validation Manager (AVM) helps companies meet compliance requirements and stay focused on core business objectives.
View More
-
Resource
How Automated Validation Manager Enables Risk-Based Compliance
See how TraceLink’s Automated Validation Manager (AVM) helps companies implement a leaner risk-based approach to software validation and meet compliance requirements.
View More
-
Resource
Automated Validation Manager: Key Features and Functionality
Learn about the key features of TraceLink Automated Validation Manager, the key to ensuring continuous validation across the lifecycle of your TraceLink solutions.
View More
-
Resource
Pharmacy Packaging is Changing. Will You Be Ready?
Will you be ready? Pharmacies are starting to recognize the potential business value of the data in the 2D barcode and data matrix. Read to see why.
View More
