Regulatory/Compliance
Thank you for contacting us; we’ll be in touch shortly.

Serialization, Onboarding Partnerships & the Hub under EU FMD
Learn from use cases that explore hub connectivity, onboarding & implementation timelines to meet compliance by February 2019.

Getting Started: EU FMD Guide to Pharma Serialization
Get started with understanding EU FMD regulations and the serialization challenges ahead, with this introductory infographic guide.

Culture of Complying at Crawford Memorial Hospital Drives Due Diligence
Leaving DSCSA details to TraceLink is a “no-brainer” for compliance-driven Crawford Memorial Hospital

Case Study: Ferrer | Building a Master Data Strategy for EU FMD
Learn how Ferrer worked with TraceLink to manage its master data for EU FMD compliance.

Case Study: IBSA | Using Serialization to Ensure Product Integrity
Learn how IBSA used serialization to protect their product from counterfeiting.

Case Study: Medreich | EU FMD from Project Plan to Post Implementation
See how Medreich and TraceLink collaborated to achieve EU FMD compliance.

Case Study: Medreich | Anti-Tampering and EU FMD
Learn how Medreich designed an EU FMD-compliant label to work with anti-tampering devices.

Case Study: Mithra | Serializing Across Multiple Business Cases
Learn how Mithra used a multidisciplinary approach for a successful EU FMD go-live.

Case Study: Noden Pharma | The Cost of Non-Compliance
See how global pharmaceuticals company Noden Pharma avoided the financial and operational risks of DSCSA noncompliance.
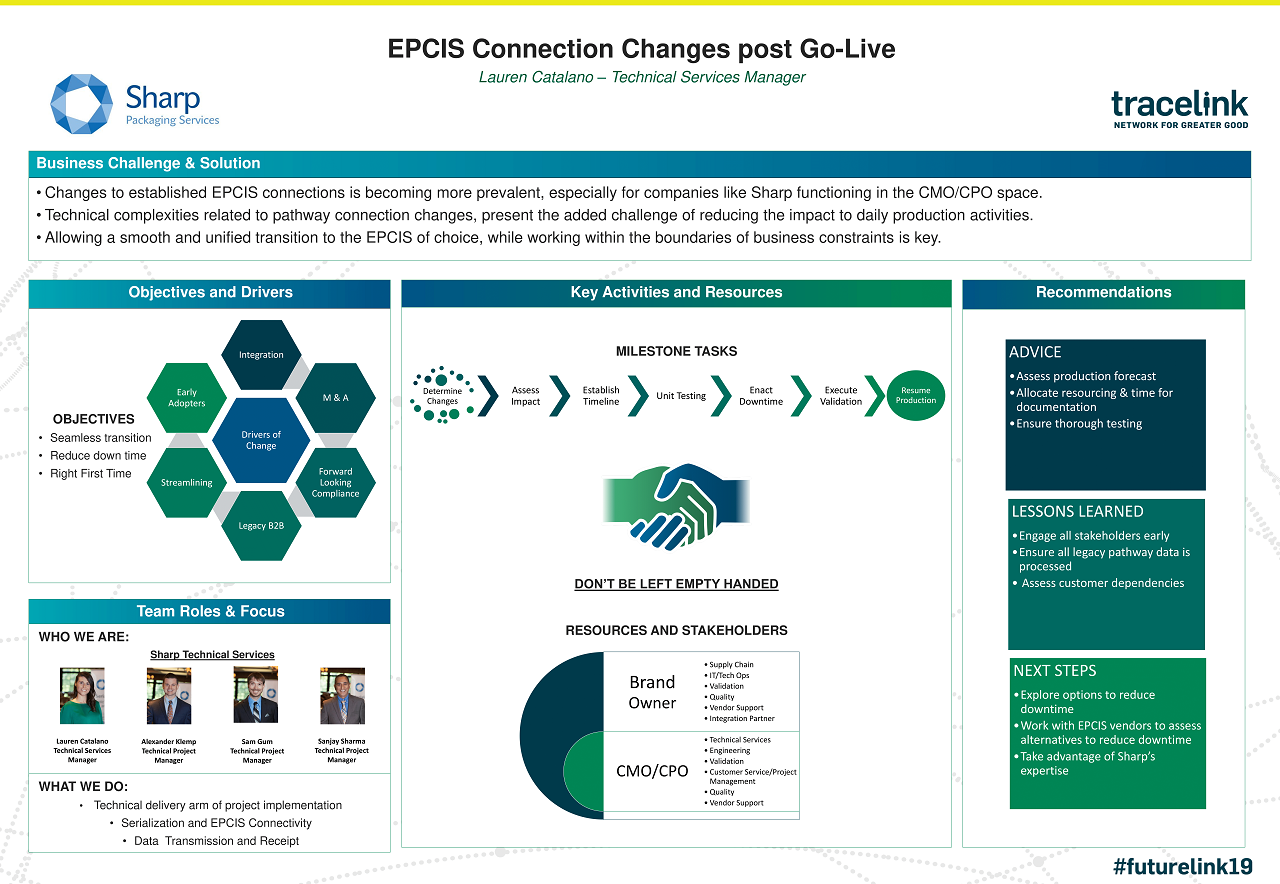
Case Study: Sharp Packaging Services | EPCIS Connection Changes Post Go-Live
See how Sharp Packaging Services overcame EPCIS change management challenges in the pharma supply chain with TraceLink's help.

Case Study: TraceLink | FDA Pilot - Product Traceability Under 2023 DSCSA Regulations - A Business Process-Led Design for a Blockchain Network
TraceLink's breakthrough blockchain solution, Trace Histories, can help pharma customers comply with US DSCSA regulations that go into effect in 2023.
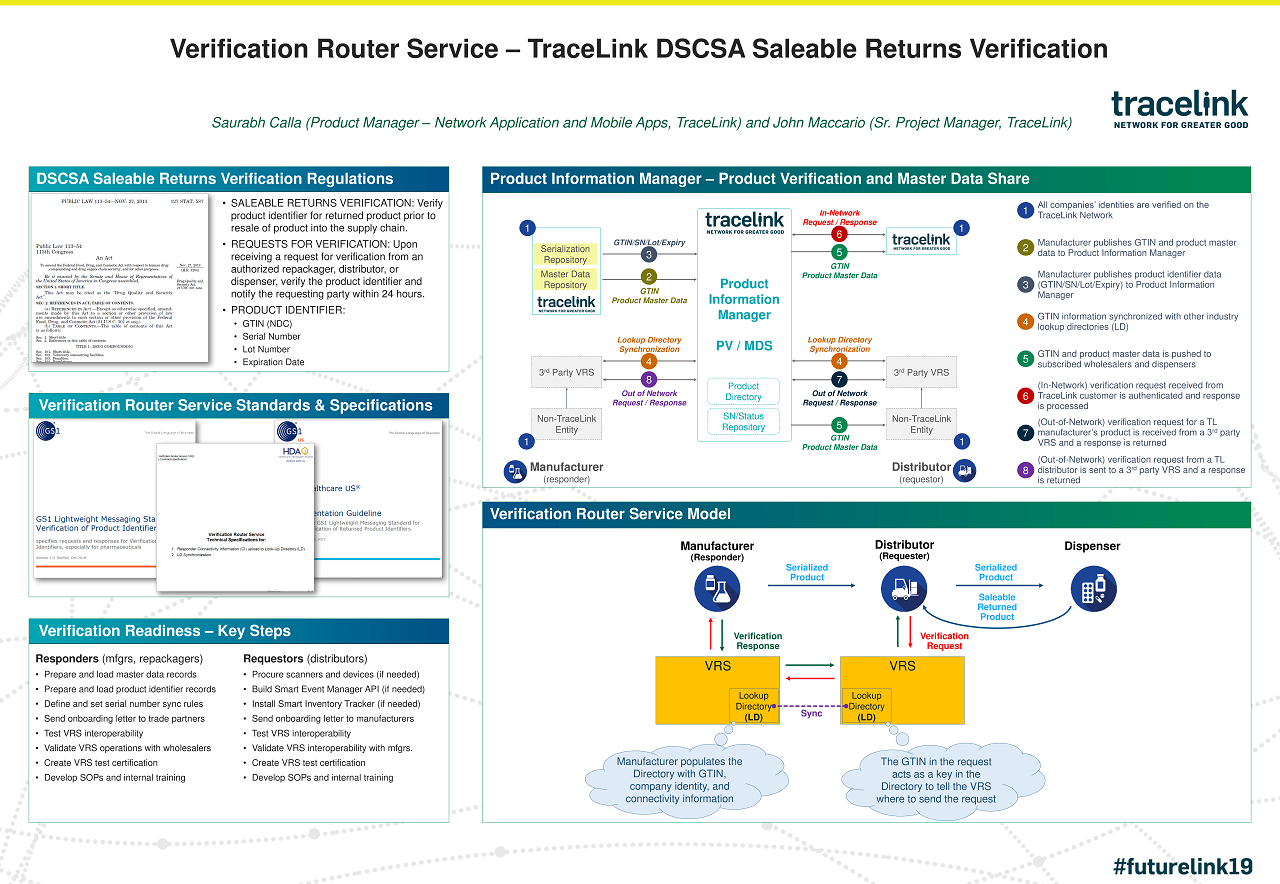
Case Study: TraceLink | Verification Router Service - TraceLink DSCSA Saleable Returns Verification
TraceLink helps customers meet DSCSA saleable returns verification requirements via the Verification Router Service model. See how.

