Regulatory/Compliance
Thank you for contacting us; we’ll be in touch shortly.
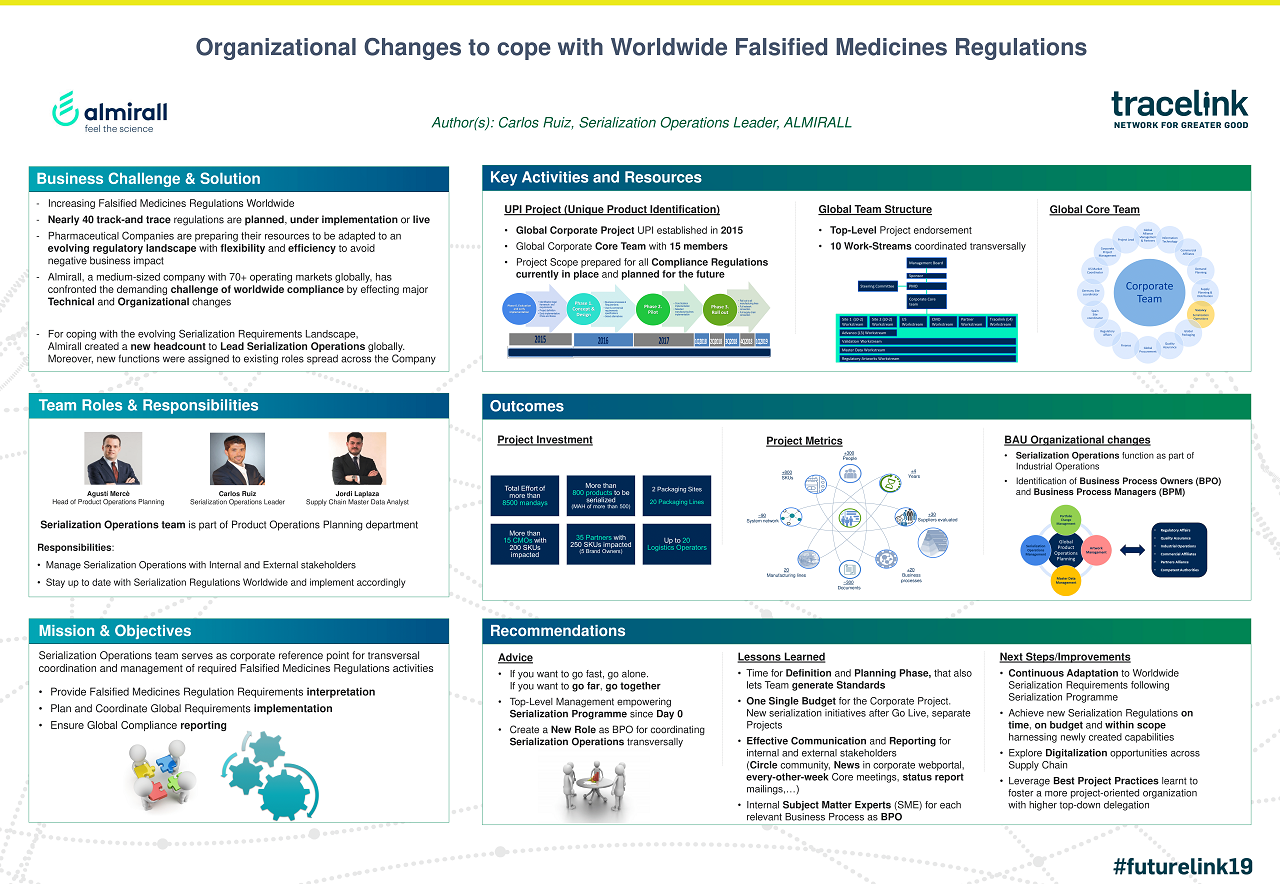
Case Study: Almirall | Organizational Changes to Cope with Worldwide Falsified Medicines Regulations
See how TraceLink helped customers like Almirall implement global compliance strategies to comply with worldwide falsified medicine regulations.

Case Study: TraceLink | Partner with TraceLink to Achieve Compliance and Highlight Your Success
Comply with global track and trace regulations and showcase pharma supply chain compliance. See how TraceLink helps.

Case Study: Value Drug Company | DSCSA Product Investigation—A Compliance Solution
See how Value Drug Company standardized the process for illegitimate and suspect product investigations for DSCSA compliance.

Serialization and Beyond: The Sharp Packaging Story
The mission of U.S. contract packager Sharp Packaging is to drive the convergence between the physical package and the digital data points as they…

FDA Issues Long-Awaited Grandfathering Guidance
Insights into the November 27, 2017, FDA draft guidance around grandfathering product under DSCSA.

FDA Issues Guidance on DSCSA Waivers, Exceptions and Exemptions
In May 2018, the FDA published new DSCSA guidance.
ACS Q&A: Building a National System for EU FMD
Read an interview with ACS PharmaProtect to learn how the German national system works and how it will operate under EU FMD.

Laetus Q&A: How People, Process, and Planning Will Drive Your EU FMD Compliance Success
Track and Trace Manager at Laetus explains why humans have a major impact on traceability, and what insights supply chain companies can gain from…

FDA Announces Enforcement Delay for Manufacturers But Law Still in Effect
Understand what the announcement means for pharma companies as the November 2017 deadline approaches.

How EU FMD Impacts CMOs
Find out in under two minutes what CMOs need to do to maintain and win business under EU FMD.

Operational Efficiency Matters: Why Knox Community Hospital Changed DSCSA Solutions
Not all DSCSA compliance solutions are created equally. That’s what Knox Community Hospital discovered the hard way, after struggling with “an…

Preparing for EU FMD & DSCSA: The Sharp Packaging Solution Perspective
Hear Sharp technical executives discuss the similarities and the critical differences between U.S. DSCSA and EU FMD.

