Table of contents
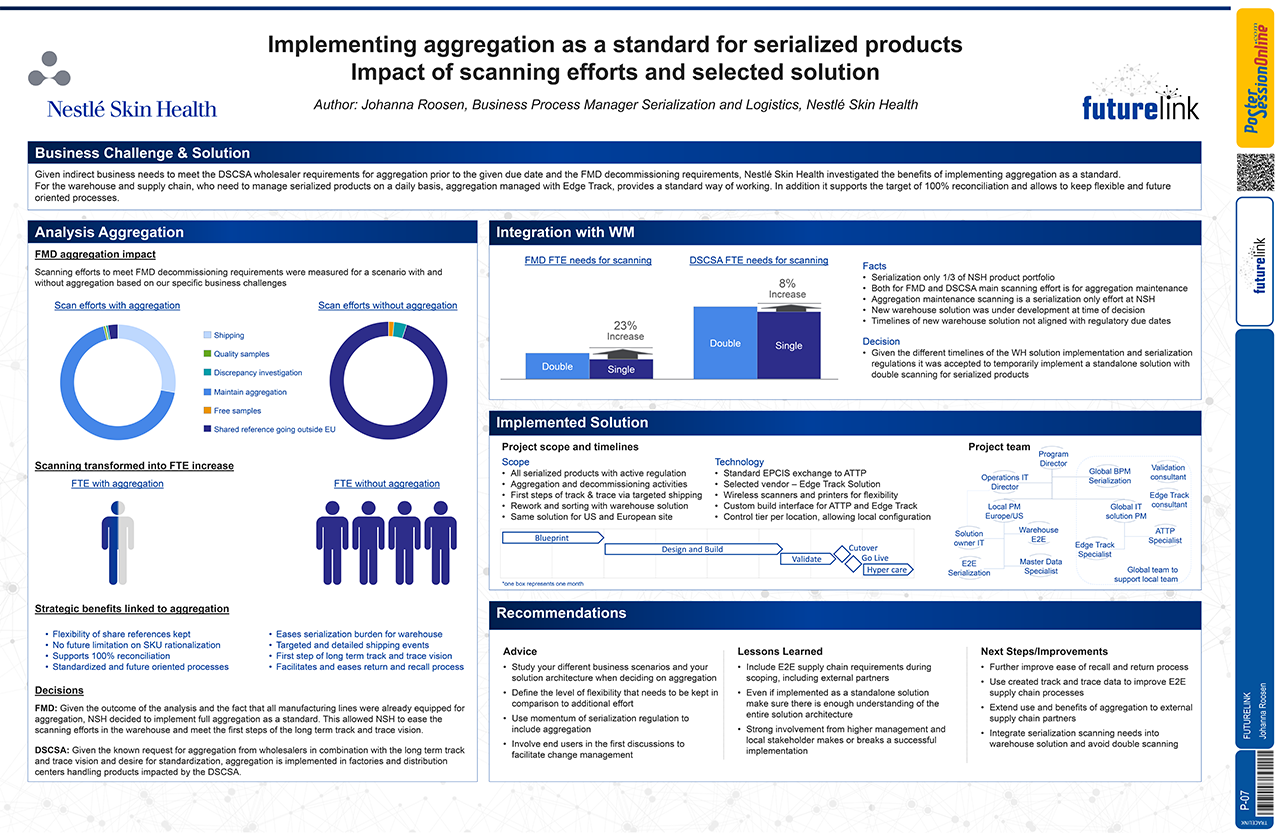
Facing both DSCSA wholesaler requirements and EU FMD decommissioning requirements, Nestlé Skin Health recognized the benefits of implementing aggregation as a standard business practice. Learn how they use a TraceLink Edge solution to support their aggregation goals, and to lay the foundation for future improvements. Nestlé Skin Health's poster, “Implementing Aggregation as a Standard for Serialized Products,” was one of two winners out of 11 featured during FutureLink Barcelona’s interactive Poster Sessions.