
-
Resource
FutureLink Nashville: Session Highlights
Get all of the highlights from FutureLink 2019 in Nashville, with insights from keynotes, general sessions, industry panels, and breakout sessions.
View More
-
Resource
India Regulatory Updates
View a compilation of the most recent track and trace regulations for the healthcare supply chain in India. Get insights into compliance updates.
View More
-
Resource
Introducing Serialized Product Intelligence: Take Control of Your Serialized Supply Chain
Learn about Santen Pharmaceutical's journey of serialization and digitization, transforming challenges into opportunities. Watch the webinar.
View More
-
Resource
Introducing TraceLink Agile Issue Management
To learn more about TraceLink's Agile Issue Management Solution for supply chain management, register for the agile supply chain webinar.
View More
-
Resource
Investing in Our Customer's Success An Update on TraceLink Services and Support Advancements
Watch this on-demand webinar to learn how TraceLink is continuously improving and investing in our customers' success.
View More
-
Resource
Russia Regulatory Updates
View a compilation of the most recent track and trace regulations for the healthcare supply chain in Russia. Get insights into compliance updates.
View More
-
Resource
Saudi Arabia Regulatory Updates
View a compilation of the most recent track and trace regulations for the healthcare supply chain in Saudi Arabia. Get insights into compliance.
View More
-
Resource
Serialized Operations: Challenges and Opportunities
How do your serialized operations compare with more 100+ pharmaceutical companies? Get the results of TraceLink's serialization program assessment.
View More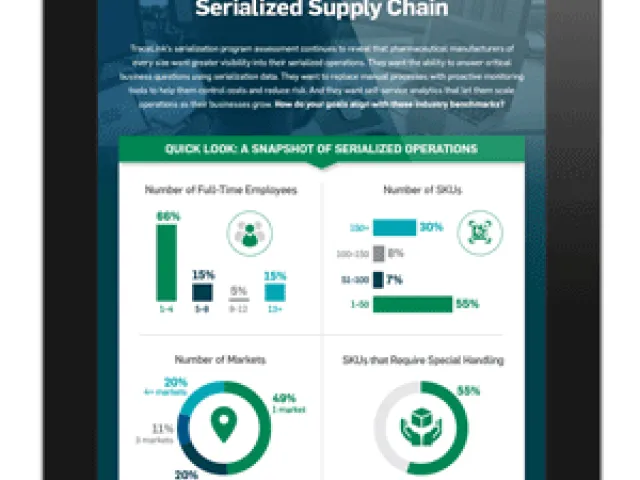
-
Resource
Solving Saleable Returns: Critical Steps Toward Meeting the Deadline
In this webinar series, TraceLink experts will guide you through the maze of saleable returns and product verification:
View More
-
Resource
Hidden Costs of Serialization
Are you prepared to navigate the entire serialization iceberg? View a breakdown of all costs you will incur.
View More
-
Resource
Operational Efficiency Matters: Why Knox Community Hospital Changed DSCSA Solutions
Not all DSCSA compliance solutions are created equally. That’s what Knox Community Hospital discovered the hard way, after struggling with “an economical vendor”.
View More
-
Resource
Preparing for EU FMD & DSCSA: The Sharp Packaging Solution Perspective
Hear Sharp technical executives discuss the similarities and the critical differences between U.S. DSCSA and EU FMD.
View More
-
Resource
Poll: 4 of 5 Phase 3s Are Preparing for Serialization Before FDA Approval
Polls show that 82% of clinical-stage virtual pharma companies, uncertain about the impact of serialization, are preparing for DSCSA compliance now.
View More
-
Resource
Starts and Stops: 6 Steps You Can Take to Increase Supply Chain Resilience
Get expert advice from IDC on how to boost pharma supply chain agility, responsiveness, and resilience.
View More
-
Resource
It’s a New Era in Supply Chain: Who Will Succeed?
Traditional pharma and healthcare supply chain operating models are changing all around us—just look at supply problems surrounding the COVID-19 pandemic to understand the reasons behind the urgent need for transformation.
View More
-
Resource
Podcast Episode 17: Bob Cantow on Why ERP is Necessary, But Not Sufficient
McKinsey & Company's Bob Cantow explains why optimizing patient outcomes requires more than just an ERP system. Watch it now.
View More
-
Resource
Podcast Episode 22: Klaus Imping on What an Agile Supply Chain Is—And What It Isn't
Klaus Imping, CEO of mSE Solutions, offers practical advice on what it takes to build an agile supply chain. Watch it now.
View More
-
Resource
Podcast Episode 15: Radhika Subramanian on Supply Chain Agility and the Benefits of Thinking Across Silos
Slalom Practice Director Radhika Subramanian offers a step-by-step guide to achieving supply chain agility. Watch it now.
View More
-
Resource
Podcast Episode 4: Steven Daugherty on Disruption as a Competitive Advantage
Get a lesson in supply chain transformation and agility from Steven Daugherty of The Kraft Heinz Company.
View More
-
Resource
Podcast Episode 2: Jake Barr on a Crawl, Walk, Run Approach to Supply Chain Transformation
Learn how Procter & Gamble developed best-in-class supply chain processes in this podcast with Jake Barr, the company’s former Global Director of Supply Network Operations.
View More
-
Resource
Special Episode: IDC on Winning Strategies for Increasing Resilience in the Face of Supply Chain Disruptions
Get expert advice from IDC on what it takes to boost supply chain resiliency and respond effectively to supply chain disruptions like those caused by COVID-19.
View More
-
Resource
Podcast Episode 13: Stuart Whiting on How Supply Chain Logistics Can Be Your Differentiator
Find out how logistics can be a key differentiator in your agile supply chain.
View More
-
Resource
Podcast Episode 3: Peter Bigelow on the Critical Role of CDMOs in Supply Chain Agility
TraceLink's Roddy Martin and senior pharmaceutical supply chain executive Peter Bigelow discuss the critical role CDMOs play in the pursuit of supply chain agility.
View More
-
Resource
The Path to Pharma Supply Chain Visibility: 5 Capabilities You Need to Focus on Now
Learn the five capabilities you’ll need to develop to achieve actionable, end-to-end supply chain visibility.
View More
-
Resource
Podcast Episode 5: Pat McLagan on Why Supply Chains Are at a Tipping Point
Get supply chain transformation expert Pat McLagan's take on why it’s past time for supply chain mental models to change.
View More
-
Resource
Podcast Episode 14: John Gattorna on Demand-Driven Segmentation in the Pharmaceutical Supply Chain
Get to know the principles of outside-in, demand-driven segmentation for the pharmaceutical supply chain.
View More
-
Resource
Podcast Episode 9: Mark Dorfmueller on the Importance of Operational Resilience
With more than three decades of supply chain and digital transformation experience at Procter & Gamble and General Electric, Mark Dorfmueller talks with TraceLink’s Roddy Martin about the importance of operational resilience.
View More
-
Resource
LogiPharma Panel: 6 Key Insights on Agility and Resilience from Global Supply Chain Thought Leaders
Get expert advice on how to achieve supply chain agility from renowned supply chain leaders.
View More
-
Resource
Podcast Episode 16: Dino Petrarolo on the Importance of Codifying the Business Improvement Journey
Learn about the science of continuous business improvement with Dino Petrarolo, Senior Vice President of Competitive Capabilities International.
View More
-
Resource
Podcast Episode 12: Omera Khan on the Upside and Downside of Supply Chain Risk
Professor Omera Khan underscores the vital importance of supply chain risk management and the sometimes surprising upside of risk, this week on The Agile Supply Chain Podcast with Roddy Martin.
View More
