Case Study
Thank you for contacting us; we’ll be in touch shortly.

Case Study: CPL | The CMO Serialization Perspective—Utilizing a Standardized Approach for Efficient Partner Onboarding
See how contract manufacturer Contract Pharmaceuticals Limited implemented a 3-step process for smooth pharmaceutical partner onboarding.

European Contract Packager Tjoapack Paves the Way for Supply Chain Efficiency
Read about contract packager Tjoapack's readiness to meet EU FMD deadlines and provide customers with an easy path to compliance.

Case Study: Value Drug Company | DSCSA Product Investigation—A Compliance Solution
See how Value Drug Company standardized the process for illegitimate and suspect product investigations for DSCSA compliance.

Case Study: TraceLink | Partner with TraceLink to Achieve Compliance and Highlight Your Success
Comply with global track and trace regulations and showcase pharma supply chain compliance. See how TraceLink helps.
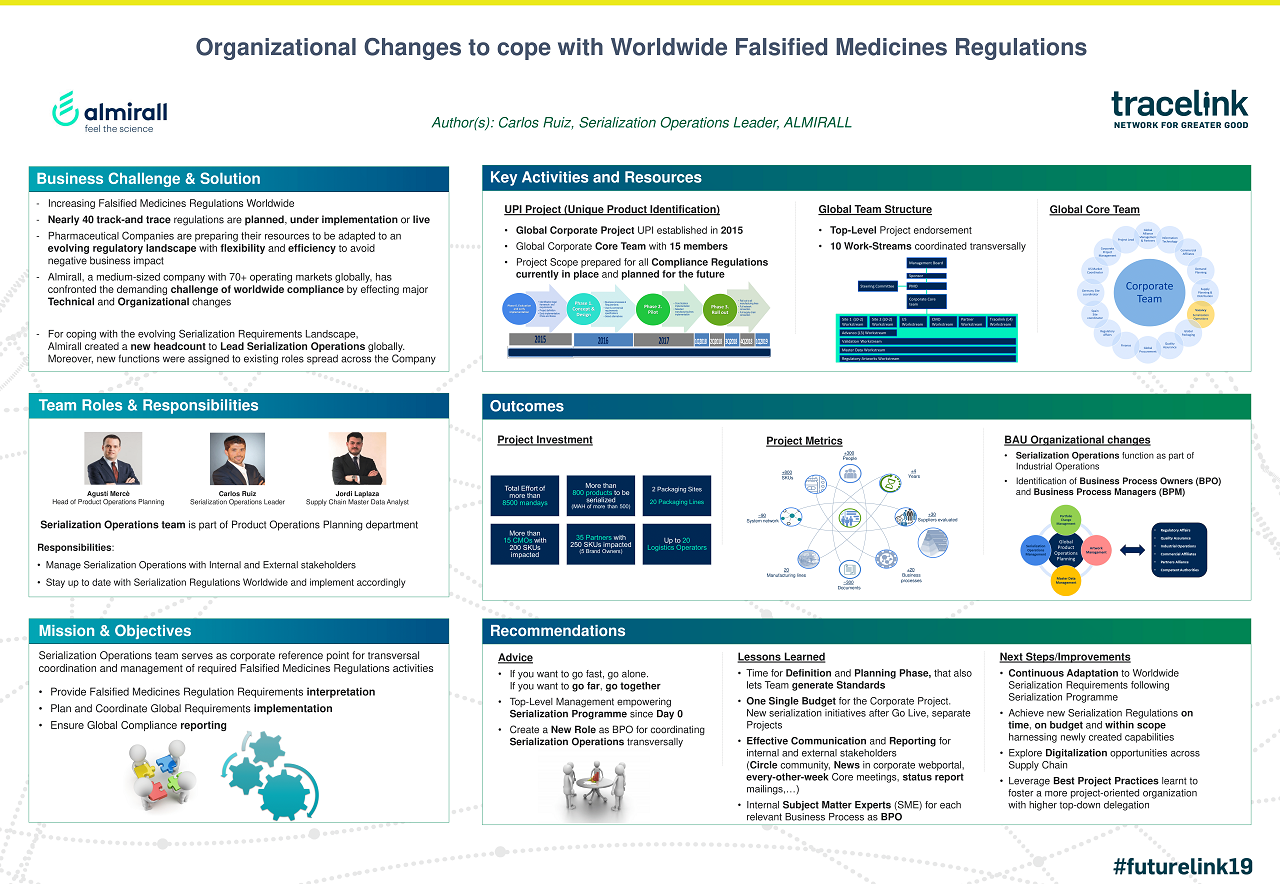
Case Study: Almirall | Organizational Changes to Cope with Worldwide Falsified Medicines Regulations
See how TraceLink helped customers like Almirall implement global compliance strategies to comply with worldwide falsified medicine regulations.
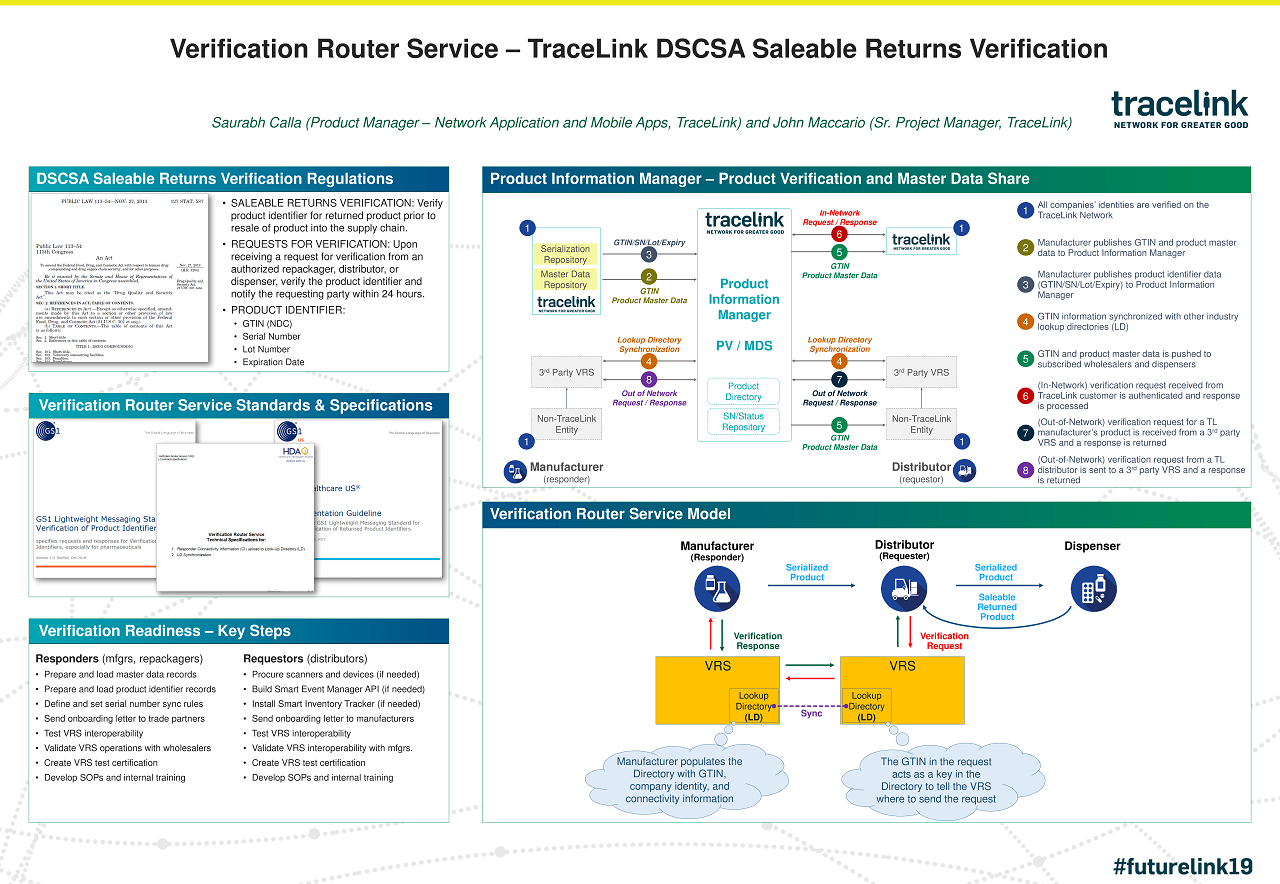
Case Study: TraceLink | Verification Router Service - TraceLink DSCSA Saleable Returns Verification
TraceLink helps customers meet DSCSA saleable returns verification requirements via the Verification Router Service model. See how.

Case Study: TraceLink | FDA Pilot - Product Traceability Under 2023 DSCSA Regulations - A Business Process-Led Design for a Blockchain Network
TraceLink's breakthrough blockchain solution, Trace Histories, can help pharma customers comply with US DSCSA regulations that go into effect in 2023.
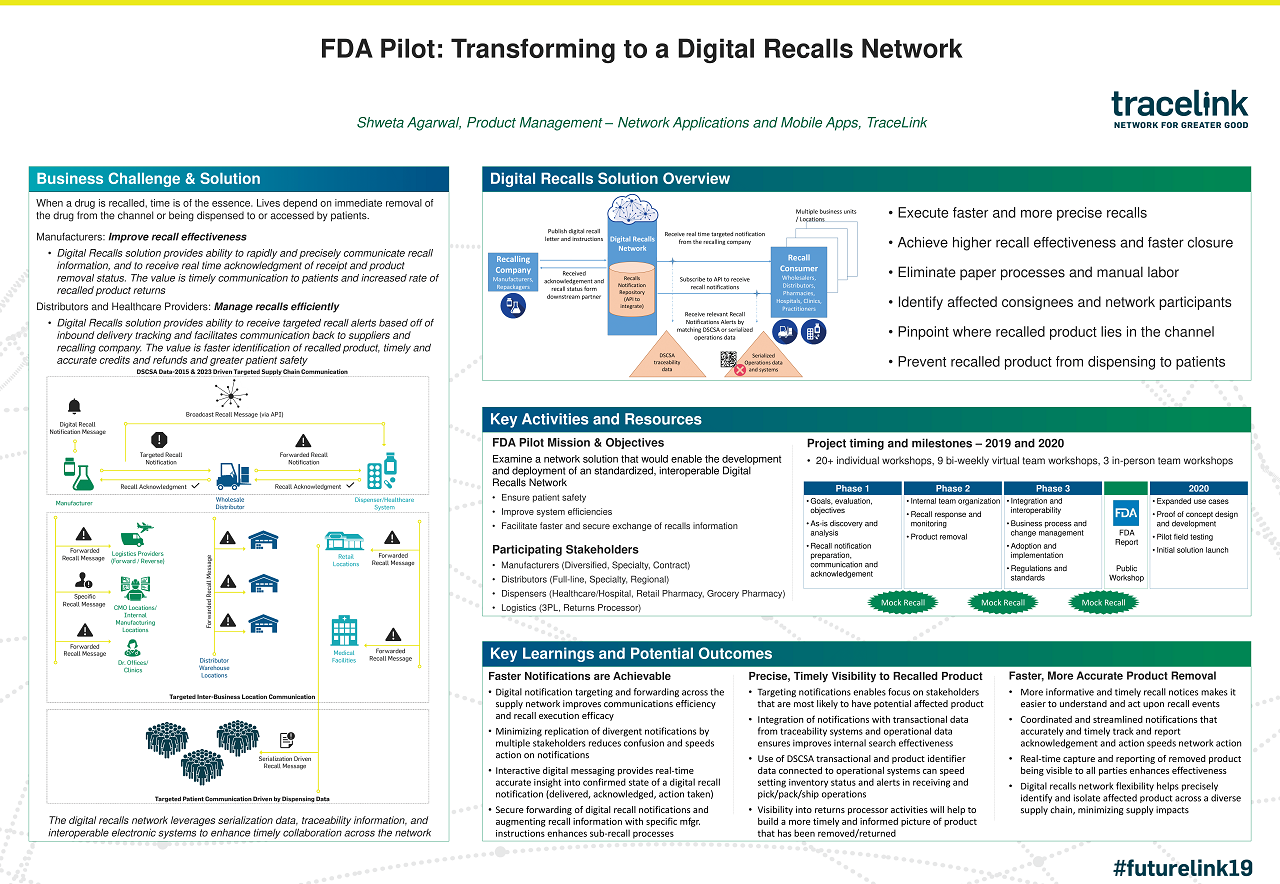
Case Study: TraceLink | FDA Pilot - Transforming to a Digital Recalls Network
Find out how TraceLink helps pharmaceutical manufacturers and dispensers manage recalls more quickly and efficiently than ever.
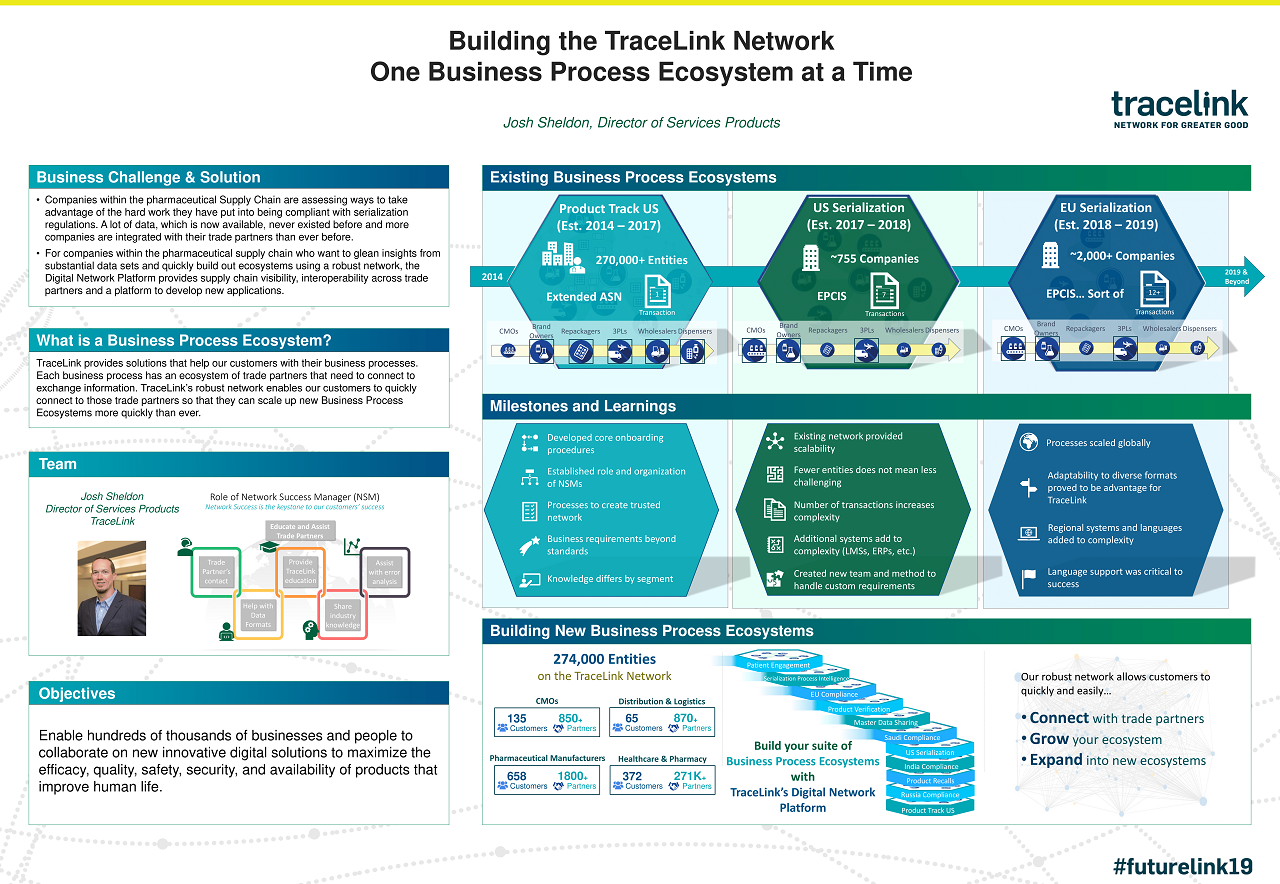
Case Study: TraceLink | Building the TraceLink Network One Business Process Ecosystem at a Time
See how TraceLink's powerful digital supply network enables customers to quickly connect to trade partners for scaling up business process ecosystems.
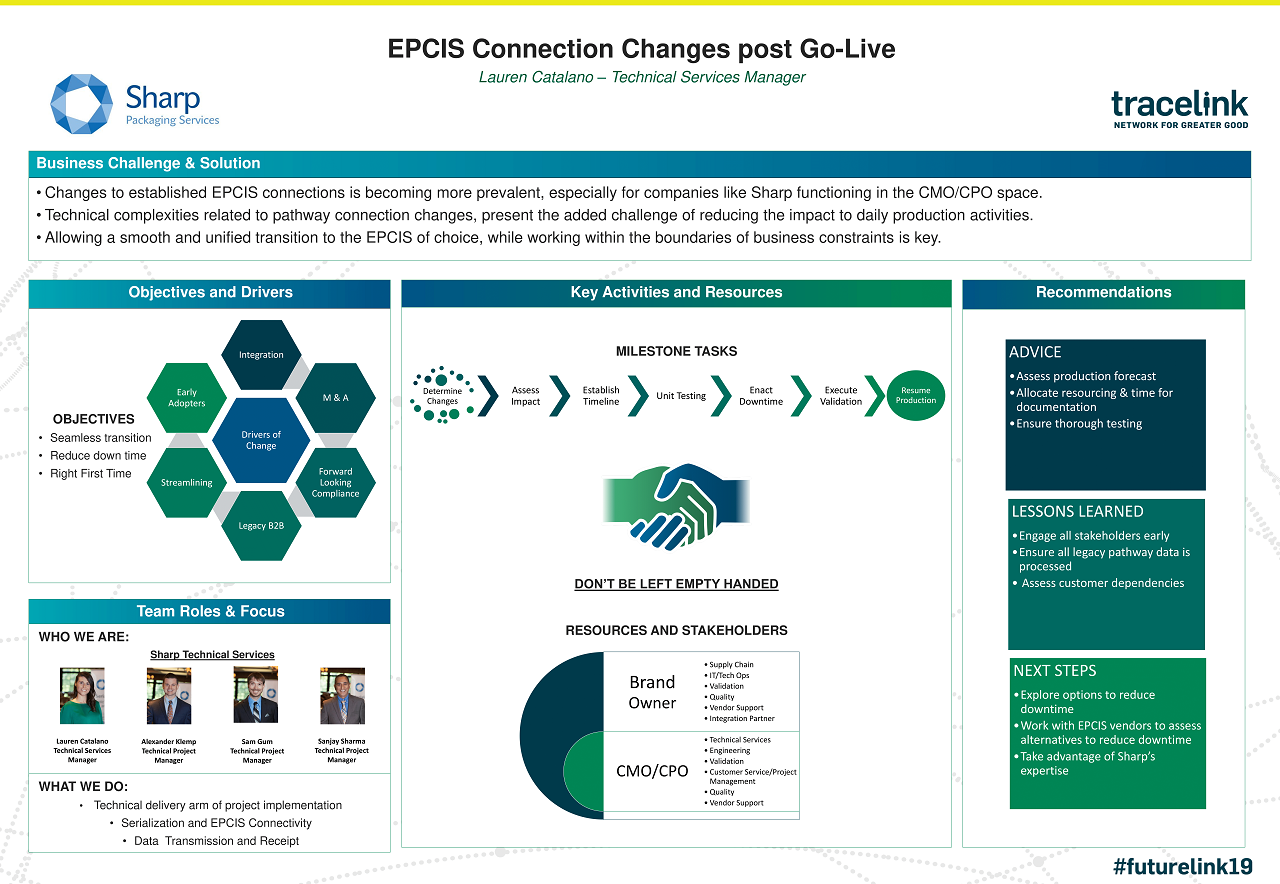
Case Study: Sharp Packaging Services | EPCIS Connection Changes Post Go-Live
See how Sharp Packaging Services overcame EPCIS change management challenges in the pharma supply chain with TraceLink's help.

Case Study: Noden Pharma | The Cost of Non-Compliance
See how global pharmaceuticals company Noden Pharma avoided the financial and operational risks of DSCSA noncompliance.

Culture of Complying at Crawford Memorial Hospital Drives Due Diligence
Leaving DSCSA details to TraceLink is a “no-brainer” for compliance-driven Crawford Memorial Hospital

