Table of contents

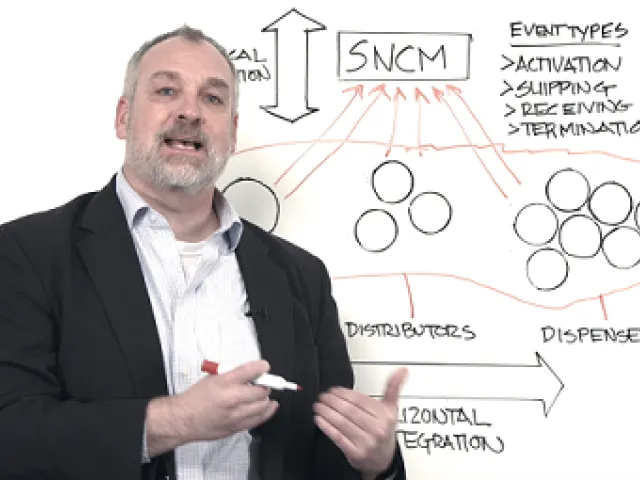
Even if you’ve serialized medicines for the U.S. and EU, the Brazil market will be a daunting challenge. Tight timelines, evolving regulations, and a country that is new to serialization require rapid mobilization and end-to-end, horizontal integration across your trade partner network.
TraceLink experts explain why Brazil may be the industry’s biggest compliance challenge, and how you can accelerate your Brazil strategy with TraceLink’s proven Integrate Once, Interoperate with Everyone™ approach to global compliance.