
-
Resource
The Supplier Quality Challenge: Improving Products and Business Processes with Multienterprise Work Management
Ensuring supplier quality is a major challenge for supplier relationship management teams. Learn how multienterprise work management can help.
View More
-
Resource
Out-of-the-Box Supply Chain Visibility with Serialized Product Intelligence
Learn how Serialized Product Intelligences gives you end-to-end visibility into your serialized supply chain without complex configurations or IT support.
View More
-
Resource
Why a Win-Win Mindset Is Important in Times of Short Supply
The experts from supply chain and procurement analyst firm Azul Partners share five tips on how to work with suppliers to build a foundation of trust, open communication, and a win-win mindset.
View More
-
Resource
Survey: Late Shipments Top List of Biggest Sources of Supply Chain Risk
Manufacturing executives reveal the top sources of supply chain risk—and what you can do about them. Get the survey results.
View More
-
Resource
Resolve supply chain issues up to 65% faster with Agile Process Teams for Supply Chain Issue Management (APT-SCIM)
Discover the supply chain issue management platform that improves visibility with real-time dashboards and enables manufacturing organizations to resolve supply chain issues up to 65% faster. View the infographic and download the eBook now.
View More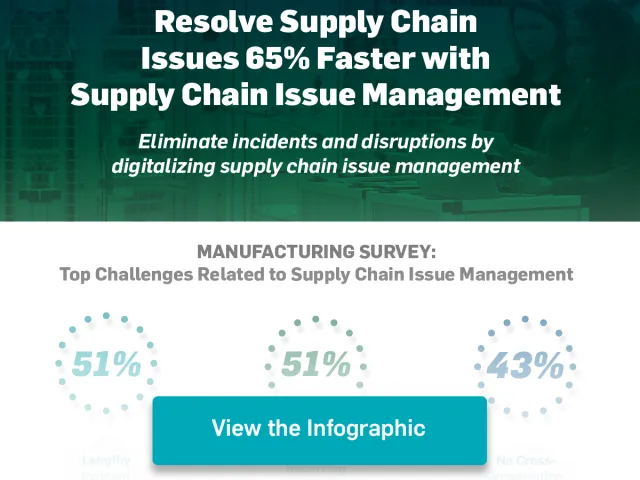
-
Events / Webinar
It’s Time for Change. Let’s Improve Supply Chain Performance
Ready to see the groundbreaking new digital technology that will fundamentally change supplier relationship management and improve supply chain performance across pharma, manufacturing and retail?
View More
-
Resource
Supply Chain Issue Management Best Practices: Your Guide to Achieving Operational Excellence Through Digitalization and Continuous Improvement
Learn how manufacturers are achieving up to 82% improvement in delivery performance; up to a 35% reduction in supply chain issues; and up to a 50% reduction in repeat deviations.
View More
-
Resource
The Top Six Supply Chain Issue Management Challenges—and How to Overcome Them
Lengthy issue resolution times top the list of the six biggest challenges to successful supply chain issue management. Get the survey results.
View More
-
Education
South Korea Compliance eLearning Course
Learning Objectives List the serialization requirements for products being sold in or exported from South Korea. Describe the role of the South Korea…
View More
-
Resource
Survey: Improving Supplier Quality is the Top Priority for Manufacturers
A whopping 84% of manufacturers say they want to improve supplier quality, but they may need to rethink the way they collaborate with supply chain partners to be successful.
View More
-
Resource
Why the Top Five Forms of Supply Chain Collaboration May Be Slowing You Down
Email and phone calls top the list of supply chain collaboration methods, but they also impede efforts to reduce disruptions. Get the survey results.
View More
-
Resource
Survey: Manufacturers Reveal Wish Lists for Improving Supply Chain Collaboration
Half of manufacturing executives wish they had a better way to monitor the performance of supply chain partners. Get the supply chain collaboration survey results.
View More
-
Resource
Establishing a Strategic Foundation for Industry 4.0
Learn how Industry 4.0 will transform the supply chain landscape forever.
View More
-
Resource
ISM June 2021 Report: Manufacturers See Increased Demand, but Struggle to Keep Up
Manufacturers across all segments are running at record volume, but constraints hinder them from producing more.
View More
-
Resource
New China Packaging Specs Point to Patient-Level Data Visibility
Learn how China’s new standards could impact how your company prepares and prints product data for the China market.
View More
-
Resource
DSCSA Governance Group Reveals 2023 Interoperability Blueprint
See why the Partnership for DSCSA Governance Blueprint is the “starting gun” for meeting DSCSA 2023 requirements.
View More
-
Resource
Brazil: ANVISA Normative Instruction Spurs Industry Action Ahead of April 2022 Deadline
See key provisions of the ANVISA Normative Instruction and what manufacturers and importers must do to meet the April 2022 deadline.
View More
-
Resource
Reduce Defects per Million by As Much As 96% with Continuous Improvement
TraceLink Agile Process Teams for Supply Chain Issue Management (APT-SCIM) provides a digital foundation for continuous process improvement through methodologies like Six Sigma and Lean by providing a systematic approach to issue management.
View More
-
Resource
Monitor, Manage, and Resolve Supply Chain Issues with Real-Time Dashboards
Agile Process Teams for Supply Chain Issue Management (APT-SCIM) gives everyone in your supply chain the power to immediately capture and report incidents.
View More
-
Resource
Reducing Supply Chain Risk Through Earlier Notification of Supply Chain Issues
When the COVID-19 pandemic started to seriously disrupt supply chains, the United States Food and Drug Administration (FDA) immediately issued guidance and asked for earlier notification of manufacturing and supply chain issues.
View More
-
Resource
Virtual Teams of Subject Matter Experts Eliminate Confusion in Supply Chain Issue Resolution
Agile Process Teams for Supply Chain Issue Management (APT-SCIM) enables you to digitally and dynamically create process teams on demand within an easy-to-use web or mobile interface.
View More
-
Resource
The Six Key Capabilities Required to Enable Faster Supply Chain Issue Resolution
“In God we trust, all others must bring data.” W. Edwards Deming, the well-known thought-leader in the field of continuous process improvement, is attributed with this famous statement about the necessity of using an analytical, data-driven approach for making decisions.
View More
-
Resource
Three Essential Capabilities That Prevent Supply Chain Issues From Escalating into Supply Chain Disruptions
Companies that have digitalized supply chain issue management have seen significant supply chain performance improvements.
View More
-
Resource
How a Major Manufacturer Improved Delivery Performance by 82% and Avoided $100M in Annual Cost
A major manufacturer embarked on a critical operational excellence initiative to manage the quality and operational performance of its suppliers, with the aim of ensuring reliable delivery of goods and services from highly capable suppliers—without interruption.
View More
-
Resource
Top 5 Problems Impacting Supply Chain Issue Resolution Processes
While supply chain teams make their best efforts to resolve issues as quickly as possible, they have not had a digital system designed specifically to facilitate structured collaboration and work management between internal teams and supply chain partners—until now.
View More
-
Resource
5 Steps to a Successful Business Process Improvement Project with APT-SCIM
Digitalize supply chain issue management with TraceLink.
View More
-
Resource
Improve Supply Chain Performance by Learning from Past Supply Chain Issues
Address the lack of institutional memory with APT-SCIM.
View More
-
Resource
Get Real-time Visibility and Reduce Supply Chain Issue Resolution Time by up to 65%
Gain real-time visibility by digitalizing issue management.
View More
-
Resource
Managing Change the Smarter Way with Agile Process Teams for Supply Chain Issue Management
Learn how to digitalize, streamline, and speed up supply chain change request processes.
View More
-
Resource
Brazil Deadline: Final Normative Instruction Means All Segments Must Comply by April 2022
With only 8 months before the deadline, learn why manufacturers need to start their Brazil compliance projects now.
View More
