
-
Resource
Investing in Our Customer's Success An Update on TraceLink Services and Support Advancements
Watch this on-demand webinar to learn how TraceLink is continuously improving and investing in our customers' success.
View More
-
Resource
Russia Regulatory Updates
View a compilation of the most recent track and trace regulations for the healthcare supply chain in Russia. Get insights into compliance updates.
View More
-
Resource
Saudi Arabia Regulatory Updates
View a compilation of the most recent track and trace regulations for the healthcare supply chain in Saudi Arabia. Get insights into compliance.
View More
-
Resource
Serialized Operations: Challenges and Opportunities
How do your serialized operations compare with more 100+ pharmaceutical companies? Get the results of TraceLink's serialization program assessment.
View More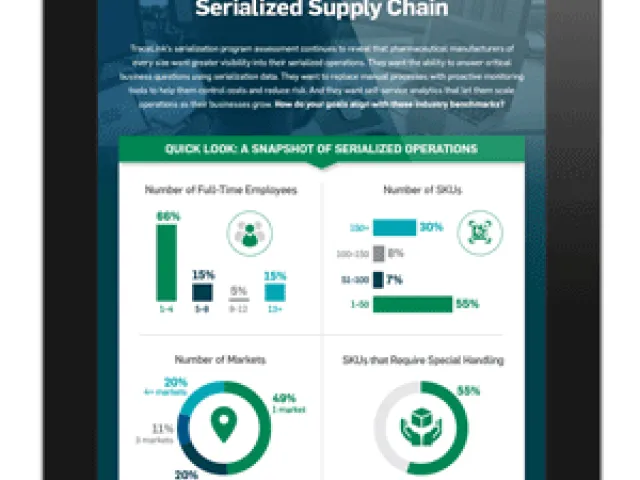
-
Resource
Solving Saleable Returns: Critical Steps Toward Meeting the Deadline
In this webinar series, TraceLink experts will guide you through the maze of saleable returns and product verification:
View More
-
Resource
Solving the COVID-19 Pharma Supply Chain Struggle
See exclusive research on COVID-19's impact on pharmaceutical supply chain agility, visibility, and resilience. Get actionable insights.
View More
-
Resource
Taking Control of Your Serialized Supply Chain An Industry Benchmark
Learn how serialization process exceptions and supply chain disruptions are impacting your peers and your own path to operational excellence.
View More
-
Resource
The End-to-End Customer Journey On-Demand Webinar: How TraceLink Supports its Customers' Success
Watch this on-demand webinar to learn more about TraceLink’s end-to-end customer journey—from solution awareness through successful implementation and value realization.
View More
-
Resource
The Only Comprehensive Analysis of the Pharmaceutical Supply Chain During the Pandemic
Watch this on-demand webinar to learn how to apply these industry-wide learnings to mitigate future disruptions in your supply chain
View More
-
Resource
The Path to Building Supply Chain Agility in Healthcare
Hear from global supply chain thought leaders as they discuss the journey to an agile, resilient, and reliable supply chain.
View More
-
Resource
Transforming the Health System Pharmacy Supply Chain Through DSCSA
Watch this on-demand webinar to learn how health system pharmacies are driving new value from compliance.
View More
-
Resource
Transforming the Retail Pharmacy Supply Chain Through DSCSA On-Demand Webinar
Watch this on-demand webinar to learn how retail pharmacies are using DSCSA as a catalyst for supply chain transformation.
View More
-
Resource
Turn Russia Compliance into a Competitive Advantage with Serialized Product Intelligence from TraceLink
Watch this on-demand webinar to learn how Serialized Product Intelligence can help you turn your serialized operations in Russia into a competitive advantage.
View More
-
Resource
United States Regulatory Updates
View a compilation of the most recent track and trace regulations for the healthcare supply chain in the U.S. Get insights into DSCSA compliance.
View More
-
Tracelink University
Article master APIs
Product or Article Master Data facilitates seamless collaboration between manufacturers and third-party logistics providers (3PLs).
View More -
Tracelink University
Carrier shipment statuses APIs
The Carrier Shipment Statuses facilitates the exchange of shipment status updates between a carrier (e.g., a trucking company or 3PL) and key stakeholders such as shippers, buyers, and other supply chain partners.
View More -
Tracelink University
Order status report APIs
Order Status Report, is a key communication tool used to improve visibility and coordination across the supply chain.
View More -
Tracelink University
Planned order APIs
A planned order is a system-generated proposal created within Enterprise Resource Planning (ERP) systems—such as SAP—or Advanced Planning Systems (APS), to signal the anticipated need to manufacture or procure a specific quantity of a pharmaceutical product or raw material at a specific time.
View More -
Tracelink University
Process order APIs
A process order is used to manage and control production in process manufacturing industries such as pharmaceuticals, chemicals, and food and beverage.
View More -
Tracelink University
Product activities APIs
The product activities report is routinely distributed to trading partners, suppliers, or upstream supply chain partners.
View More -
Tracelink University
Routing carrier instructions APIs
The routing and carrier instructions are essential in the transportation and shipping industry, providing detailed guidelines to ensure efficient and compliant movement of goods.
View More -
Tracelink University
Order status reports
A Order Status Report allows suppliers and CMOs to stay aligned with customer expectations, proactively communicate any delays or issues, and help ensure compliance with contractual and service-level commitments.
View More -
Tracelink University
Routing and carrier instructions
The routing and carrier instructions transaction is used in the logistics and transportation industry to deliver detailed routing instructions, ensuring the efficient shipment and delivery of products from carriers to shippers.
View More -
Tracelink University
Product transfer account adjustment requests
A Product Transfer Account Adjustment Request is a formal transaction used in the pharmaceutical supply chain to correct financial discrepancies related to the movement of products between trading partners—typically between wholesalers/distributors and manufacturers.
View More -
Tracelink University
Product transfer account adjustment responses
A Product Transfer Account Adjustment Response is a notification from a manufacturer or supplier that lets you know the outcome of an adjustment request—usually related to a chargeback from a wholesaler or distributor.
View More -
Press Release / News
TraceLink Co-Founder and SVP of Supply Network Products Lucy Deus Named Recipient of 2024 Women in Supply Chain Award
TraceLink, the only no-code platform for intelligent orchestration of end-to-end supply chains, proudly announces that Lucy Deus, SVP of Supply Network Products at TraceLink, has been named a winner of Supply & Demand Chain Executive’s 2024 Women in Supply Chain Award
View More
-
Tracelink University
What's new in the OPUS Reports and Dashboards, version 2024.1.0 release
Explore what's new in the OPUS Reports and Dashboards, version 2024.1.0 release.
View More -
Resource
Orchestrating Outcomes for External Manufacturing: Arun Giddu of Thermo Fisher Scientific on Laying the Groundwork for Long-Term ROI in Supply Chain Digitalization - Part 1
Arun Giddu, Director of Supply Chain at Thermo Fisher Scientific, explains why a well-implemented ERP system and a robust layer of master data are critical to achieving ROI on supply chain digitalization initiatives. Watch now!
View More
-
Resource
Orchestrating Outcomes for External Manufacturing: Arun Giddu of Thermo Fisher Scientific on Laying the Groundwork for Long-Term ROI in Supply Chain Digitalization - Part 2
Arun Giddu, Director of Supply Chain at Thermo Fisher Scientific, provides a step-by-step guide on how to measure the success of a supply chain digitalization initiative. Watch now!
View More
-
Resource
FutureLink Barcelona 2024 Highlights: Intelligent Orchestration of Your End-to-End Supply Chain
Over 200 leaders from across the global life sciences and healthcare supply chain joined together at FutureLink Barcelona 2024 to develop actionable plans to digitalize critical business processes with partners. Watch the video for highlights!
View More

