
-
Partner
Conval Group
CONVAL Group was incorporated in Toronto Canada in 2000 as a company with a mission of serving as consultants and technology enablers to Life…
View More
-
Partner
Converge Consulting
TraceLink and Converge Consulting: Helping Clients Achieve Their DSCSA Goals Converge Consulting is a business consulting firm focused on…
View More
-
Resource
Improving Patient Care Through Supply Chain Digitalization: Why Boston Medical Center Chose TraceLink
Learn how Boston Medical Center is leveraging the TraceLink network to digitalize its supply chain and predict drug shortages before they impact patients. Watch the video!
View More
-
Resource
Enhance Pharmacy Inventory Management by Operationalizing DSCSA Compliance & Reverse Logistics
See how integrated solutions from TraceLink, Tecsys, and PharmaLink provide DSCSA compliance while unlocking new opportunities for operational improvement.
View More
-
Resource
Driving Change in Healthcare: Inside Boston Medical Center's Digital Supply Chain Transformation
Learn how Boston Medical Center's digital supply chain transformation will impact patient care and how TraceLink is helping. Watch this in-depth interview today.
View More
-
Partner
Infoteck Solutions
At Infoteck Solutions, we are committed to delivering top-notch IT services and IT consulting solutions tailored to meet the client unique business…
View More
-

-
Resource
Fuel Your Enterprise Procurement System with Real-Time, End-to-End Supply Chain Information
Learn how to supercharge spend management systems through cost-effective integration with all of your suppliers in three easy steps.
View More
-
Resource
Orchestrating Outcomes for Logistics: Guy Courtin of Tecsys Inc. on the Impact of Supply Chain Digitalization – Part 1
Guy Courtin of Tecsys Inc. discusses the importance of data accuracy, the "Amazon effect" on healthcare logistics, and the challenges of digitalization in a constantly changing supply chain environment. Watch the video!
View More
-
Resource
Orchestrating Outcomes for Logistics: Guy Courtin of Tecsys Inc. on the Impact of Supply Chain Digitalization – Part 2
Guy Courtin of Tecsys Inc. shares insights on outsourcing, DSCSA, warehouse automation, the Internet of Things, and their impact on supply chain digitalization initiatives. Watch the video!
View More
-
Press Release / News
TraceLink Becomes First Solution Provider to Secure All 16 GS1 US Conformance Trustmarks for EPCIS File Support
TraceLink announces it has become the first and only solution provider to secure all 16 GS1 US Conformance Trustmarks for EPCIS file support.
View More
-
Resource
Bright Visionaries, Bold Innovators: Introducing FutureLink Barcelona's Speakers
FutureLink Barcelona is coming this fall! See who will be delivering deep supply chain insight and best practices for the life sciences and healthcare supply chain conference.
View More
-
Events / Webinar
The First No-Code Platform Ecosystem for Supply Chain Management: The Future is Now for Solution, Technology, & Service Partners
The First No-Code Platform Ecosystem for Supply Chain Management: The Future is Now for Solution, Technology, & Service Partners.
View More
-
Press Release / News
TraceLink Launches Program to Enable Solution Partners and Integrators to Digitalize End-to-End Supply Chains
TraceLink Inc., the only no-code platform for intelligent orchestration of end-to-end supply chains, has announced the second generation and expansion of its ecosystem partner program, OPUS PartnerLink.
View More
-
Events / Webinar
Maximize Pharmacy Efficiency: Achieve DSCSA Compliance for 340B Inventory Without Disrupting Operations
Managing 340B product in compliance with the U.S. Drug Supply Chain Security Act (DSCSA) is complex. No matter how your organization manages 340B product, TraceLink offers the flexibility you need to meet the DSCSA compliance requirements without disruption.
View More
-
Press Release / News
TraceLink's Magnum Release of its OPUS Platform Will Revolutionize Supply Chain Management by Enabling All Businesses to Digitalize their End-to-End Supply Networks
TraceLink, the largest end-to-end digital network platform for intelligent orchestration of the life sciences and healthcare supply chain, has announced the Magnum release of its flagship platform, OPUS, the only no-code network digitalization platform designed to democratize access to end-to-end supply chain integration and orchestration.
View More
-
Resource
From the ‘Amazon Effect’ on Healthcare Logistics to the Urgent Need for Data Quality: 3 Top Trends in Supply Chain Digitalization
Get the top three takeaways from our Orchestrating Outcomes interview with Guy Courtin, Vice President of Industry & Global Alliances at Tecsys. Read the blog and watch the interview for more information!
View More
-
Resource
Maximize Pharmacy Efficiency: Achieve DSCSA Compliance for 340B Inventory Without Disrupting Operations
See how manufacturers and 3PLs can get the real-time visibility they need to achieve best-in-class on-time, in-full customer shipment performance. Watch the webinar now!
View More
-
-
Tracelink University
Understanding Page Types within the OPUS Solution Environment (OSE)
Page types enable Solution Designers to efficiently create user-friendly pages using a drag-and-drop interface, allowing them to organize information for optimal usability.
View More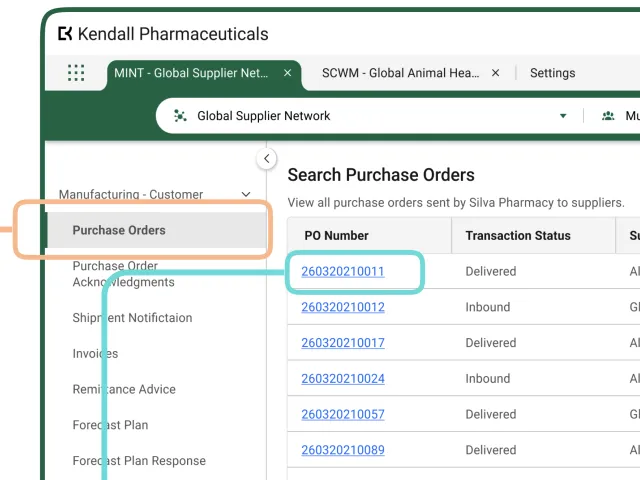
-
Tracelink University
Custom XTT Link Actions Development Guide
Walkthrough of developing new XTT Link Actions.
View More
-

-
Resource
Be a Featured Guest on Orchestrating Outcomes!
Become a featured guest on Orchestrating Outcomes, a new video series from TraceLink focused on supply chain digitalization. Learn more!
View More
-
Press Release / News
TraceLink's OPUS Link Actions Minimize Cost and Time to Integrate Enterprise System APIs with Your End-to-End Digitalization Strategy
TraceLink, the largest end-to-end digital network platform for intelligent orchestration of the supply chain, has announced OPUS Link Actions, pre-built API (Application Programming Interface) integrations that link the OPUS Platform and its growing suite of multi-enterprise solutions with an expanding list of ERP and enterprise systems — the first release of which is for NetSuite.
View More
-
Resource
Orchestrating Outcomes for Logistics: Dan Bell of Marken on Driving Digitalization and Patient-Centric Innovation in Pharma Supply Chains – Part 1
Dan Bell, Senior Vice President of Innovation and Strategic Operations at Marken, shares insights on how Marken leverages UPS’s vast global network to ensure patient safety and product quality. Watch the interview!
View More
-
Resource
Orchestrating Outcomes for Logistics: Dan Bell of Marken on Driving Digitalization and Patient-Centric Innovation in Pharma Supply Chains – Part 2
Dan Bell, Senior Vice President of Innovation and Strategic Operations at Marken, explains why building resilience into every link of the supply chain is the key to driving both precision and progress. Watch the interview!
View More
-
Tracelink University
UX Writing and Terminology
The goal is to create UI text that is clear and concise, offering users the essential information they need to effectively complete their tasks.
View More
-
Tracelink University
Use Case: XTT Link Actions
Integrate with external systems using Link Actions.
View More
-
Tracelink University
Custom Transforms Development Guide
Walkthrough of how to create your own custom transforms.
View More
-
Tracelink University
XTT Link Actions Developer Kit
Configure your development environment.
View More

